Understanding how solvents affect nucleophilicity is crucial in organic chemistry. Nucleophilicity refers to the ability of a molecule or ion to donate a pair of electrons to form a new chemical bond. Solvents can significantly impact this property.
I. Introduction to Nucleophilicity
Nucleophiles are substances that are rich in electrons. They can donate them to an electron-deficient center, usually a carbon atom. Also, the strength of a nucleophile can be influenced by the solvent it is in.
A. Polar Protic Solvents
Polar protic solvents have a hydrogen atom attached to an electronegative atom like oxygen or nitrogen.
Examples:
- Water (H₂O): A commonly used solvent that can form hydrogen bonds.
- Methanol (CH₃OH): Another polar protic solvent used in many reaction
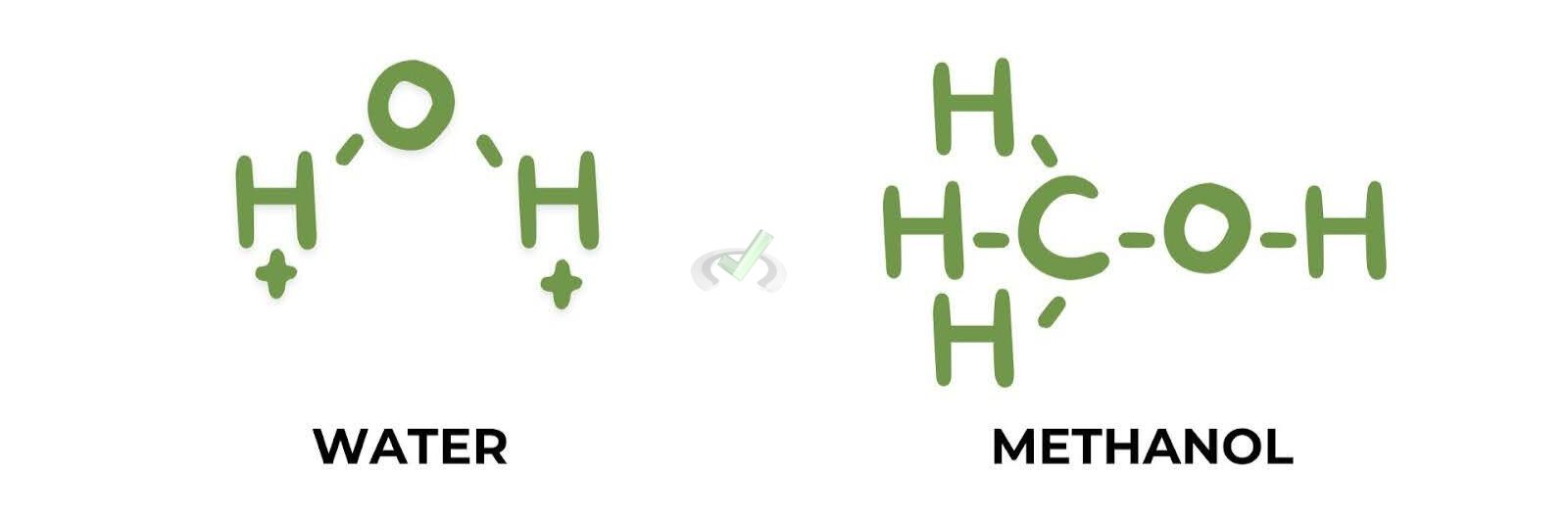
These solvents can form hydrogen bonds with nucleophiles. This makes the nucleophiles less nucleophilic. The hydrogen bonding reduces the nucleophile's ability to donate electrons.
B. Polar Aprotic Solvents
Polar aprotic solvents do not have hydrogen atoms attached to electronegative atoms.
Examples:
Examples:
- Acetone (CH₃COCH₃): Does not form hydrogen bonds with nucleophiles, so it does not hinder their nucleophilicity.
- Dimethyl Sulfoxide (DMSO, (CH₃)₂SO): Excellent solvent for increasing nucleophilicity due to the absence of hydrogen bonding.
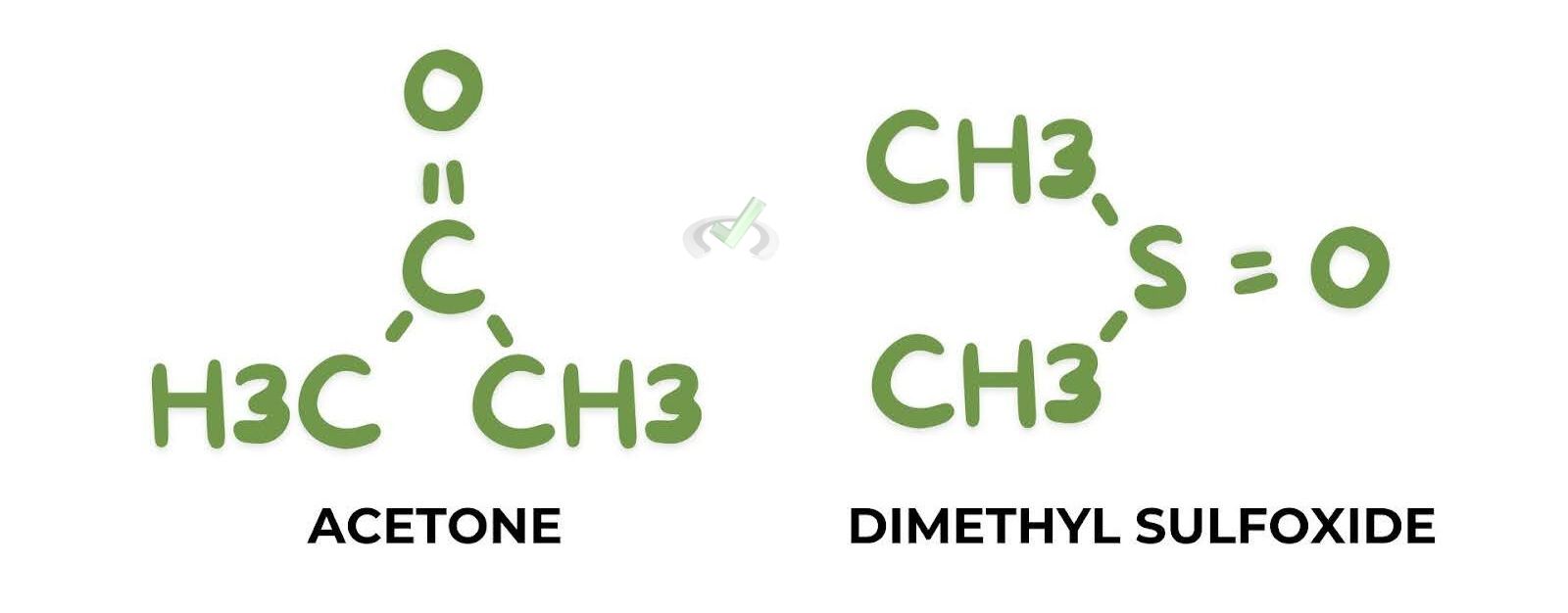
Polar aprotic solvents do not form hydrogen bonds with nucleophiles. This allows them to remain more nucleophilic compared to when they are in polar protic solvents.
II. Detailed Effects of Solvents
A. Influence of Solvent Type on Nucleophilicity
The type of solvent can change how nucleophiles behave in a reaction.
In Polar Protic Solvents
Nucleophiles are usually less effective because they form hydrogen bonds with the solvent. For example, iodide (I⁻) is a strong nucleophile in polar protic solvents. This is because it is less affected by hydrogen bonding compared to smaller anions.
Smaller anions like fluoride (F⁻) are heavily solvated due to strong hydrogen bonds. This makes them less available to participate in nucleophilic attacks.

In Polar Aprotic Solvents
Nucleophiles are more effective. Here, smaller ions like fluoride (F⁻) are very nucleophilic. This is because there are no hydrogen bonds to hinder them.
In the absence of hydrogen bonding, the anions are not solvated. They also remain free to attack electrophilic centers.

B. Practical Examples of Solvents
Understanding solvent effects can help predict reaction outcomes.
- Sodium Iodide in Acetone: In this polar aprotic solvent, iodide is a strong nucleophile and can readily participate in nucleophilic substitution reactions.
- Sodium Fluoride in Water: In this polar protic solvent, fluoride is less nucleophilic due to strong hydrogen bonding with water molecules.
III. Connecting to Broader Organic Chemistry Concepts
A. Reaction Mechanisms
Knowing how solvents affect nucleophilicity is crucial to understanding reaction mechanisms. For example, a strong nucleophile is needed in the SN2 reaction mechanism.
Choosing a polar aprotic solvent can enhance the nucleophilicity of the reactant. This leads to a faster reaction.
B. Synthesis and Practical Applications
Selecting the appropriate solvent in organic synthesis can make or break a reaction. For example, if a strong nucleophile is required, choosing a polar aprotic solvent can ensure the reaction proceeds efficiently.
In synthesizing pharmaceuticals, reactions often require precise control of nucleophilicity to achieve high yields and purity. Polar aprotic solvents like DMSO are commonly used to facilitate nucleophilic substitutions in complex molecules.
C. Biological Chemistry
In biochemistry, enzyme active sites often mimic solvent environments. Solvent effects affect nucleophilic reactions in metabolic pathways. Understanding solvent effects helps in studying enzyme mechanisms and metabolic pathways.
IV. Solvent Effects and Beyond
Grasping how solvents impact nucleophilicity helps predict and control reaction outcomes. Hence, this is essential for successful synthesis and mechanism analysis.
- Acid-Base Chemistry: The behavior of acids and bases in different solvents can influence nucleophilicity. For instance, the solvent's ability to stabilize ions is crucial in acid-base reactions.
- Thermodynamics and Kinetics: Solvent effects can influence the energy landscape of a reaction. It is fundamental in both the thermodynamic stability and the kinetic pathway.
- Biological Chemistry: In biochemistry, enzyme active sites often mimic solvent environments. It affects nucleophilic reactions in metabolic pathways.
V. Wrap-Up and Key Terms and Concepts
Understanding the effects of solvents on nucleophilicity involves grasping key concepts and principles. Let's review:
- Nucleophilicity: The ability of a molecule or ion to donate electrons.
- Polar Protic Solvent: A solvent that can form hydrogen bonds, reducing nucleophilicity.
- Polar Aprotic Solvent: A solvent that does not form hydrogen bonds, enhancing
VI. Practice Questions
Sample Practice Question 1
Which solvents would best enhance the nucleophilicity of a chloride ion (Cl⁻) in an SN2 reaction?
A) Water
B) Methanol
C) Acetone
D) Ethanol
Ans. C
Acetone is a polar aprotic solvent that does not form hydrogen bonds with nucleophiles. This enhances the nucleophilicity of chloride ions for SN2 reactions.
Sample Practice Question 2
In which type of solvent would you expect a nucleophilic substitution reaction with a fluoride ion (F⁻) to proceed most slowly?
A) Dimethyl Sulfoxide (DMSO)
B) Acetone
C) Water
D) Tetrahydrofuran (THF)
Ans. C
Water is a polar protic solvent that forms strong hydrogen bonds with fluoride ions. This reduces their nucleophilicity and slowing the reaction.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot