Redox reactions involve the transfer of electrons between molecules. The term "redox" is short for reduction-oxidation.
Reduction means gaining electrons, and oxidation means losing electrons. These reactions are crucial for many processes, including metabolism and industrial chemical production.
I. Introduction to Redox Reactions
Redox reactions play a crucial role in organic chemistry. They involve two simultaneous processes: oxidation and reduction. Let's break down these concepts.
Oxidation
Oxidation is the process where a molecule loses electrons. It often involves gaining oxygen or losing hydrogen.
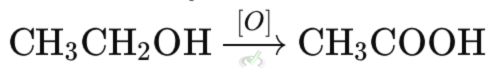
For example, when ethanol (C₂H₅OH) turns into acetic acid (CH₃COOH), it loses electrons and gains oxygen.
Reduction
Reduction is the process where a molecule gains electrons. It often involves losing oxygen or gaining hydrogen.
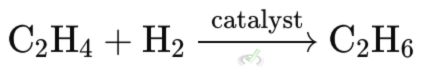
For example, when ethene (C₂H₄) becomes ethane (C₂H₆). It gains electrons and hydrogen.
II. Common Types of Redox Reactions
There are several types of redox reactions commonly found in organic chemistry. Here are a few important ones to know:
A. Combustion Reactions
These reactions involve oxygen and produce energy through heat and light.
Example: The combustion of methane (CH₄) produces carbon dioxide (CO₂) and water (H₂O). This reaction releases a lot of energy, so it is used as fuel.
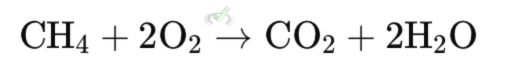
B. Hydrogenation Reactions
These reactions add hydrogen to a molecule, reducing it.
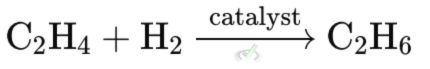
Example: Hydrogenating ethene (C₂H₄) to form ethane (C₂H₆). This reaction is used in the food industry to turn liquid oils into solid fats.
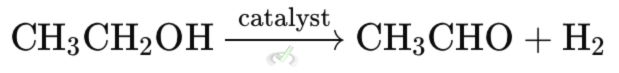
C. Dehydrogenation Reactions
These reactions remove hydrogen from a molecule, oxidizing it.
Example: Dehydrogenating ethanol (C₂H₅OH) to form acetaldehyde (CH₃CHO) is a process used to make some types of chemicals.
III. Important Reagents and Catalysts
Specific reagents and catalysts are frequently used to drive redox reactions in organic chemistry. Here are some key ones:
A. Oxidizing Agents
- Potassium Permanganate (KMnO₄): A strong oxidizing agent that oxidizes alcohols to carboxylic acids.
- Chromic Acid (H₂CrO₄): Used to oxidize primary alcohols to carboxylic acids and secondary alcohols to ketones.
- Hydrogen Peroxide (H₂O₂): A versatile oxidizing agent in various oxidation reactions, including converting sulfides to sulfoxides.
- Osmium Tetroxide (OsO₄): Used to oxidize alkenes to diols, especially useful in the dihydroxylation of alkenes.
B. Reducing Agents
- Lithium Aluminum Hydride (LiAlH₄): A powerful reducing agent used to reduce carboxylic acids, esters, and amides to alcohols.
- Sodium Borohydride (NaBH₄): A milder reducing agent used to reduce aldehydes and ketones to alcohols.
- Hydrogen Gas (H₂): Often used with a metal catalyst (such as palladium or platinum) to reduce alkenes and alkynes to alkanes.
- Diborane (B₂H₆): Used to reduce carboxylic acids and esters to alcohols and is particularly useful in hydroboration-oxidation reactions.
C. Catalysts
- Lithium Aluminum Hydride (LiAlH₄): A powerful reducing agent used to reduce carboxylic acids, esters, and amides to alcohols.
- Sodium Borohydride (NaBH₄): A milder reducing agent used to reduce aldehydes and ketones to alcohols.
- Hydrogen Gas (H₂): Often used with a metal catalyst (such as palladium or platinum) to reduce alkenes and alkynes to alkanes.
- Diborane (B₂H₆): Used to reduce carboxylic acids and esters to alcohols and is particularly useful in hydroboration-oxidation reactions.
IV. Mechanisms of Redox Reactions
Understanding the mechanisms of redox reactions helps predict the products and control the reactions. Here are some basic mechanisms:
A. Electron Transfer Mechanism
Includes the direct transfer of electrons from the reducing agent to the oxidizing agent.
Example: Reduction of copper(II) ion (Cu²⁺) to copper metal (Cu) using zinc (Zn). Here, zinc donates electrons to copper ions, turning them into solid copper.
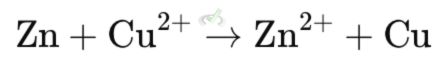
B. Hydride Transfer Mechanism
Involves the transfer of a hydride ion (H⁻) from the reducing agent to the substrate.
Example: Reduction of ketones to alcohols using sodium borohydride (NaBH₄). The hydride ion from NaBH₄ adds to the carbonyl group in the ketone, forming an alcohol.
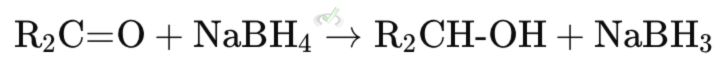
C. Disproportionation Reactions
These are special redox reactions where one element in a single compound is simultaneously oxidized and reduced.
Example: The reaction of hydrogen peroxide (H₂O₂) to form water (H₂O) and oxygen (O₂). One oxygen atom is reduced (gains electrons), and the other is oxidized (loses electrons).
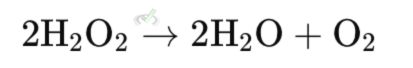
V. Applications and Examples
Redox reactions are essential in various applications, from biological systems to industrial processes. Here are a few examples:
A. Metabolism
Cellular respiration involves the oxidation of glucose (C₆H₁₂O₆) to produce energy, carbon dioxide (CO₂), and water (H₂O). This process is crucial for providing energy to cells.
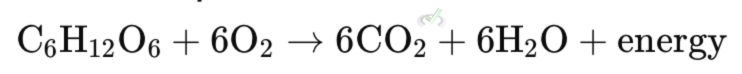
B. Industrial Synthesis
The Haber process produces ammonia (NH₃) from nitrogen (N₂) and hydrogen (H₂), involving both reduction and oxidation steps. This reaction is important for making fertilizers.

C. Environmental Chemistry
The oxidation of pollutants like sulfur dioxide (SO₂) to less harmful substances like sulfate (SO₄²⁻) in the atmosphere. This helps reduce air pollution and acid rain.
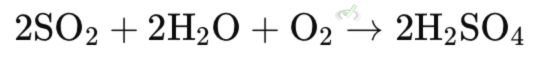
VI. Connecting to Broader Organic Chemistry Concepts
Understanding redox reactions is fundamental to grasping broader topics in organic chemistry. Here are some connections:
A. Functional Group Transformations
Redox reactions are used to transform functional groups. For example, oxidizing alcohols to aldehydes or carboxylic acids is a common transformation in organic synthesis.
B. Reaction Mechanisms
Knowledge of redox reactions helps us understand the mechanisms of various organic reactions, including synthesis and decomposition. For instance, knowing how electron transfer works can explain why some reactions happen faster than others.
C. Biochemical Relevance
Many biochemical processes, such as cellular respiration and photosynthesis, are based on redox reactions. For example, in cellular respiration, glucose is oxidized to produce energy. In photosynthesis, water is split to provide electrons for converting carbon dioxide into glucose.
VII. Wrap-Up and Key Terms
Understanding redox reactions in organic chemistry involves mastering several key concepts and terms. Let's review:
Key Terms
- Oxidation: Loss of electrons, oxygen gain, or hydrogen loss.
- Reduction: Gain of electrons, oxygen loss, or hydrogen gain.
- Oxidizing Agents: Substances that accept electrons and get reduced.
- Reducing Agents: Substances that donate electrons and get oxidized.
- Electron Transfer Mechanism: Direct transfer of electrons.
- Hydride Transfer Mechanism: Transfer of a hydride ion.
- Disproportionation Reactions: Redox reactions where one element is both oxidized and reduced.
VIII. Practice Questions
Sample Practice Question 1
What is the oxidizing agent in the oxidation of ethanol (C₂H₅OH) to acetic acid (CH₃COOH)?
A) Sodium Borohydride
B) Potassium Permanganate
C) Lithium Aluminum Hydride
D) Nickel
Ans. B
Potassium permanganate (KMnO₄) is a strong oxidizing agent that can oxidize ethanol to acetic acid. In this reaction, ethanol loses electrons (is oxidized), and potassium permanganate gains electrons (is reduced).
Sample Practice Question 2
Which of the following is a reduction reaction?
A) Combustion of methane
B) Oxidation of ethanol
C) Hydrogenation of ethene
D) Dehydrogenation of ethane
Ans. C
Hydrogenation of ethene (C₂H₄) to form ethane (C₂H₆) is a reduction reaction because ethene gains hydrogen (and electrons) during the process, reducing it to ethane.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot