Understanding the basic structure and naming rules for alcohols and ethers is important in organic chemistry. Alcohols and ethers are key functional groups. They play big roles in both biological processes and industrial uses.
I. Basics of Alcohols and Ethers
A. Structure of Alcohols
Alcohols are organic compounds having one or more hydroxyl (-OH) groups that are attached to a carbon atom. The general formula for an alcohol is R-OH, where R stands for an alkyl group (a group derived from an alkane by removing one hydrogen atom).
Example: Ethanol (C₂H₅OH) is a simple alcohol with this structure: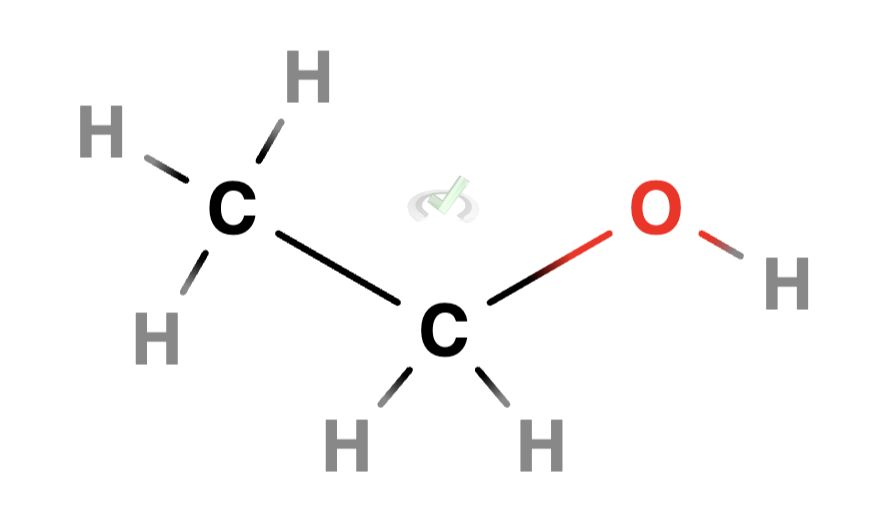
B. Structure of Ethers
Ethers are organic compounds with an oxygen atom connected to two alkyl or aryl groups. R-O-R' is the general formula for an ether. Where R and R' can be the same or in different alkyl or aryl groups.
Example: Ethanol (C₂H₅OH) is a simple alcohol with this structure: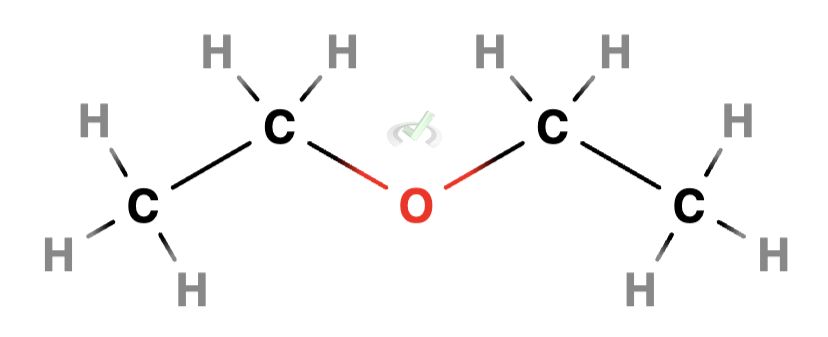
II. Nomenclature of Alcohols and Ethers
A. Naming Alcohols
- Identify the longest carbon chain containing the hydroxyl group.
- Number the chain starting from the end closest to the hydroxyl group.
- Replace the suffix of the parent alkane name with "-ol."
- Indicate the position of the hydroxyl group with a number.
Example: Propanol (C₃H₇OH)
The longest chain has three carbons (propane). The hydroxyl group is at the first carbon, so the name is 1-propanol.
B. Naming Ethers
- Name the two alkyl groups that are attached to the oxygen in alphabetical order.
- Add the word "ether" at the end.
Example: Ethyl methyl ether (C₂H₅-O-CH₃)
The two groups are ethyl and methyl. Since "ethyl" comes before "methyl" alphabetically, the name is ethyl methyl ether.
III. Common Alcohols and Ethers
A. Methanol (CH₃OH)
- Simplest alcohol
- Used as a solvent and antifreeze
Equation:
B. Ethanol (C₂H₅OH)
- Found in alcoholic beverages
- Used as a solvent and fuel
Equation:
C. Diethyl Ether (C₂H₅-O-C₂H₅)
- Commonly used as a solvent
- Historically used as an anesthetic
IV. Chemical Properties
A. Reactions of Alcohols
Dehydration to form alkenes: Alcohols can lose water (H₂O) to form alkenes.
Example:
Oxidation to form aldehydes or carboxylic acids: Primary alcohols can be oxidized to aldehydes. This can further oxidize to carboxylic acids.
Example:
B. Reactions of Ethers
Cleavage by acids: Ethers can be cleaved by strong acids to form alkyl halides and alcohols.
Example:
V. Applications of Alcohols and Ethers
A. Biological Systems
Alcohols are found in many natural substances, such as sugars and amino acids. Ethers can be used as solvents for fats, oils, waxes, perfumes, resins, dyes, gums, and hydrocarbons.
B. Industrial Processes
Alcohols are used in the production of chemicals, pharmaceuticals, and as fuels. Ethers are used as solvents and in the manufacture of chemicals.
VI. Bridge to Other Topics in Organic Chemistry
Understanding alcohols and ethers helps study other parts of organic chemistry and biochemistry.
A. Metabolism
Alcohols, like ethanol, are metabolized in the body. This means they are broken down into other compounds. For example, ethanol is converted to acetaldehyde and then to acetic acid in the liver.
B. Enzyme Reactions
Enzymes are proteins that accelerate chemical reactions in the body. Alcohols can affect how enzymes work. For instance, ethanol can inhibit enzymes that are important for metabolism.
C. Synthesis in Chemistry
Alcohols and ethers are used to make other chemicals. For example, an alcohol can be converted into an ester, another type of organic compound, through a reaction with an acid.
D. Drug Design
Many drugs contain alcohol or ether groups. Understanding their chemistry helps in designing and synthesizing new medications.
VII. Wrap-Up and Key Terms
Key Terms:
- Alcohol: Organic compound with an -OH group.
- Ether: Organic compound with an oxygen atom bonded to two alkyl or aryl groups.
- Hydroxyl group (-OH): Functional group in alcohols.
- Alkyl group: Group derived from an alkane by removing one hydrogen atom.
VIII. Practice Questions
Sample Practice Question 1
What is the general formula for an alcohol?
A. R-O-R'
B. R-OH
C. R-COOH
D. R-NH₂
Ans. B
The general formula for an alcohol is R-OH. Here, R is an alkyl group.
Sample Practice Question 2
What is the product of the oxidation of ethanol (C₂H₅OH)?
A. Methanol (CH₃OH)
B. Ethylene (C₂H₄)
C. Acetaldehyde (CH₃CHO)
D. Acetic acid (CH₃COOH)
Ans. C
The primary oxidation product of ethanol is acetaldehyde.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot