Redox reactions are a key topic in organic chemistry.
“Red-” refers to the reduction process, and “-od” refers to the oxidation process.
Both phenomena happen simultaneously, which makes sense!
The mnemonic “OIL RIG” can be used to remember how redox reactions work.
“Oxidation Is the Loss of electrons, and Reduction Is the Gain of electrons.”
The same concept can be used for redox reactions on aldehydes and ketones! Let’s consider ethanol oxidation into acetaldehyde using the oxidizing agent PCC.
1. When ethanol is oxidized, it loses electrons.
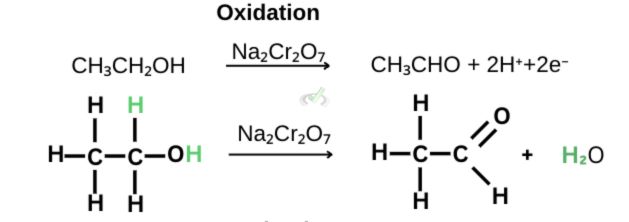
The half-reaction shown above shows that in a reaction with the oxidizing agent sodium dichromate (Na₂Cr₂O₇), ethanol turns into ethanal as it loses 2 electrons and 2 hydrogen atoms.
2. The oxidizing agent initiates the oxidation. In doing so, it undergoes reduction by accepting the electrons ethanol loses.

These reactions occur hand in hand–when something is oxidized, something must also be reduced!
Oxidation States
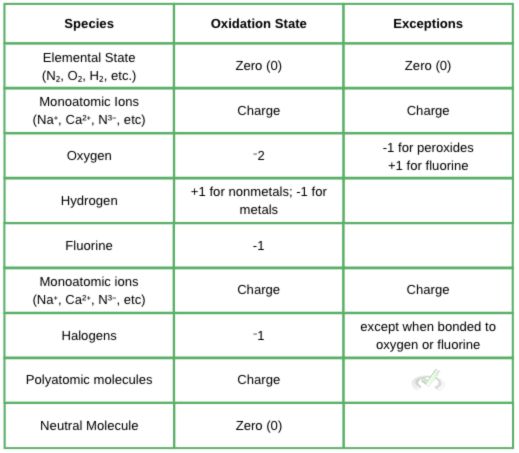
Oxidation is defined as the increase in oxidation state. The oxidation state is a number that can show the loss or gain of electrons. They are handy in redox reactions since they act as a way to see the checks and balances of electrons.
Assigning oxidation states can get quite confusing. For now, here are a few pointers to remember.
I. Oxidation and Reduction on Alcohols
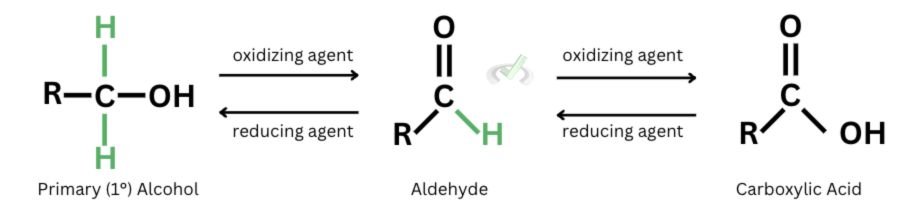
A. When primary alcohols oxidize, they form aldehydes. Further oxidation of aldehydes will form carboxylic acids. During oxidation, the oxidized species loses electrons, represented by the removal of hydrogen atoms. Since primary alcohols have two hydrogen atoms, they can get oxidized twice.
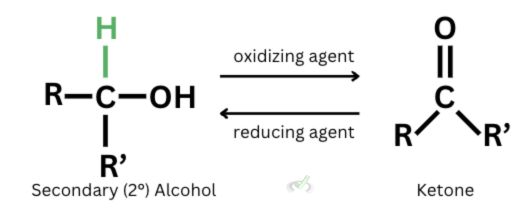
B. Secondary alcohols oxidize to form ketones. Since they only have one hydrogen atom, they can only get oxidized once.
C. Tertiary alcohols cannot undergo oxidation. They do not have hydrogen bonded to a central carbon atom. This indicates that they do not have an electron/hydrogen to lose.
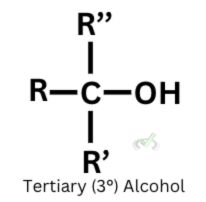
Since reduction is the reverse of oxidation, carboxylic acids can reduce into aldehydes, and aldehydes can reduce back into primary alcohols.
Oxidation is the process of losing electrons or a bond. When oxidation occurs, a compound's oxidation state increases.
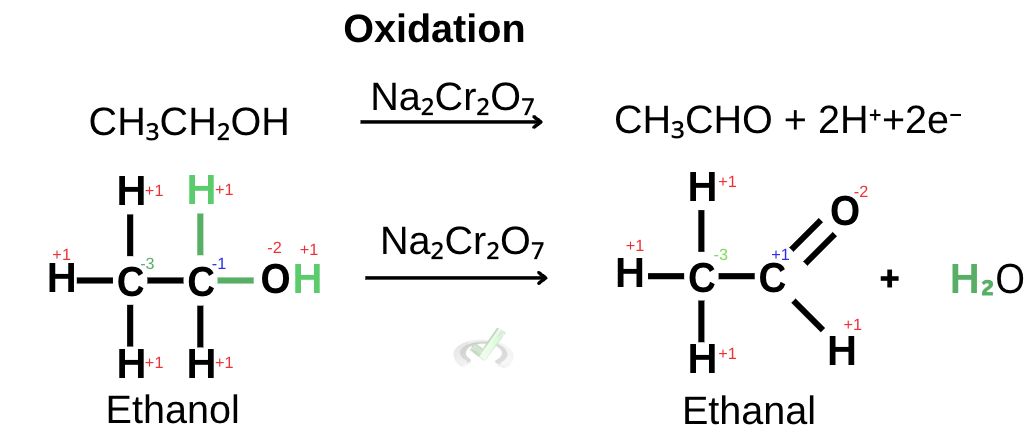
In the previous example, we saw that two hydrogen atoms were removed along with two electrons. The oxidation state of the carbon attached to the hydroxyl group has an oxidation number of -1. Once it oxidizes into ethanal, the carbon has an oxidation number of +1. This increase from an oxidation number of -1 to +1 is an indication of oxidation.
At the same time, during reduction, electrons are gained, and the oxidation state decreases.
II. Oxidizing and Reducing Agents
Redox reactions happen with oxidizing and reducing agents.
An oxidizing agent accepts electrons from a substance, resulting in the oxidation of the said substance.
A reducing agent donates electrons, resulting in the reduction of the oxidizing agent.
Knowing about common oxidizing and reducing agents during the MCAT is helpful. Oxidizing agents are generally rich in oxygen since they deliver oxygen to the reactant. They trigger oxidation and get reduced in the process. They are also poor in hydrogen. Oxidation agents will have a high oxidation state and high electron affinity.
Reducing agents, on the other hand, are rich in hydrogen and poor in oxygen. Since they initiate reduction, they get oxidized in the process. They have a low oxidation state and ionization energy. Here is a list of common reducing and oxidizing agents:
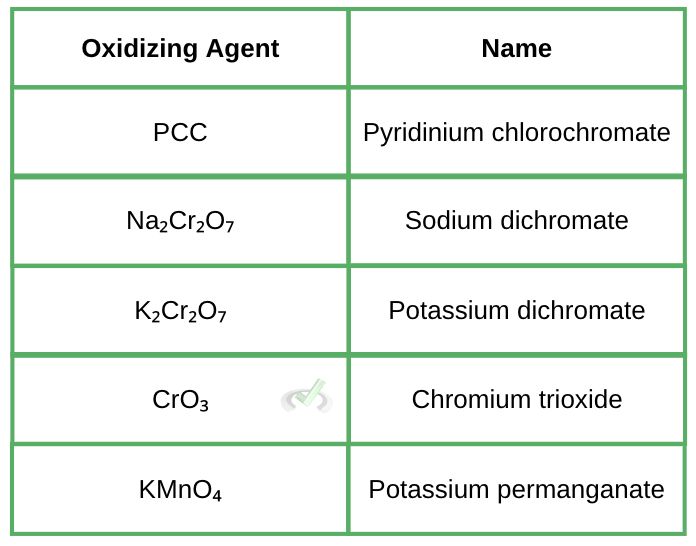
III. Worked Examples
A. Oxidation of isopropanol to propanone
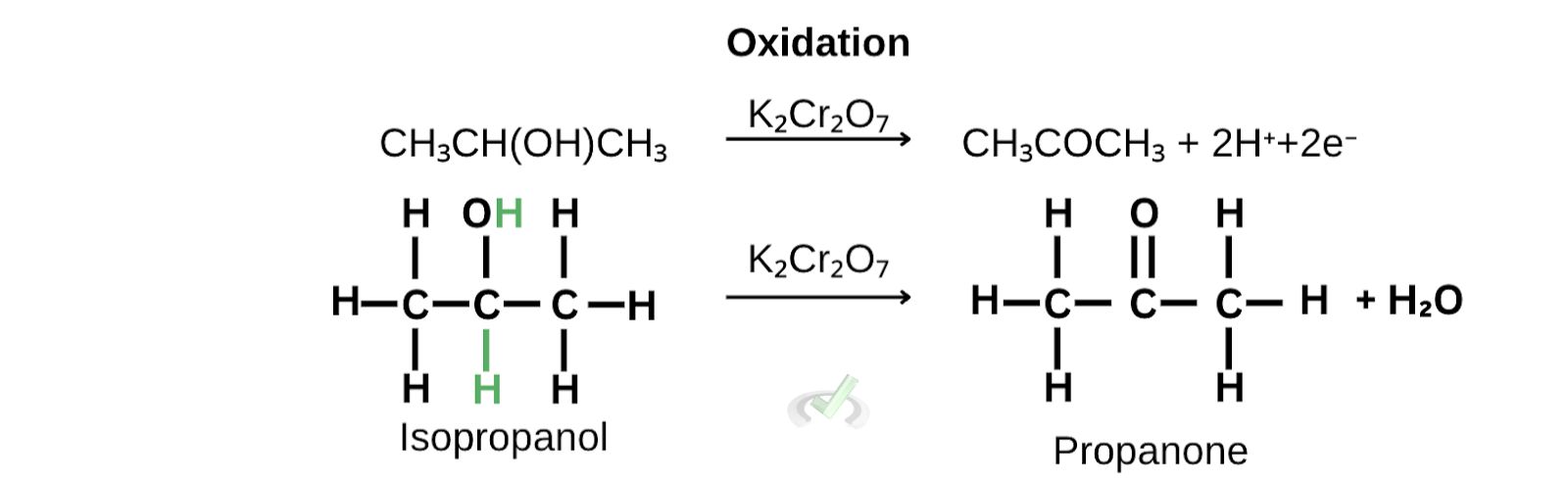
As isopropanol is oxidized, the carbon attached to the hydroxyl group loses 2 electrons, removing two hydrogen atoms.

At the same time, chromate gains electrons and forms water and chromium (III) ions.

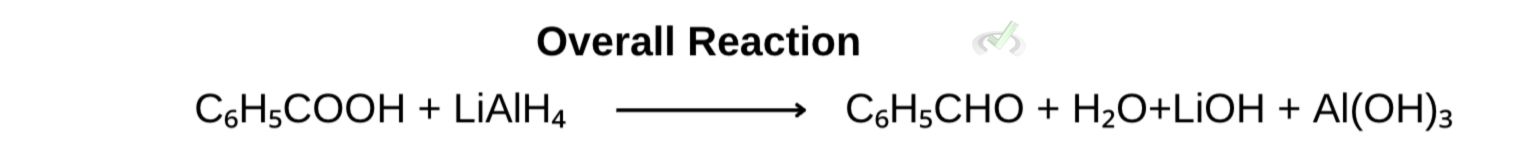
B. Reduction of benzoic acid to benzaldehyde
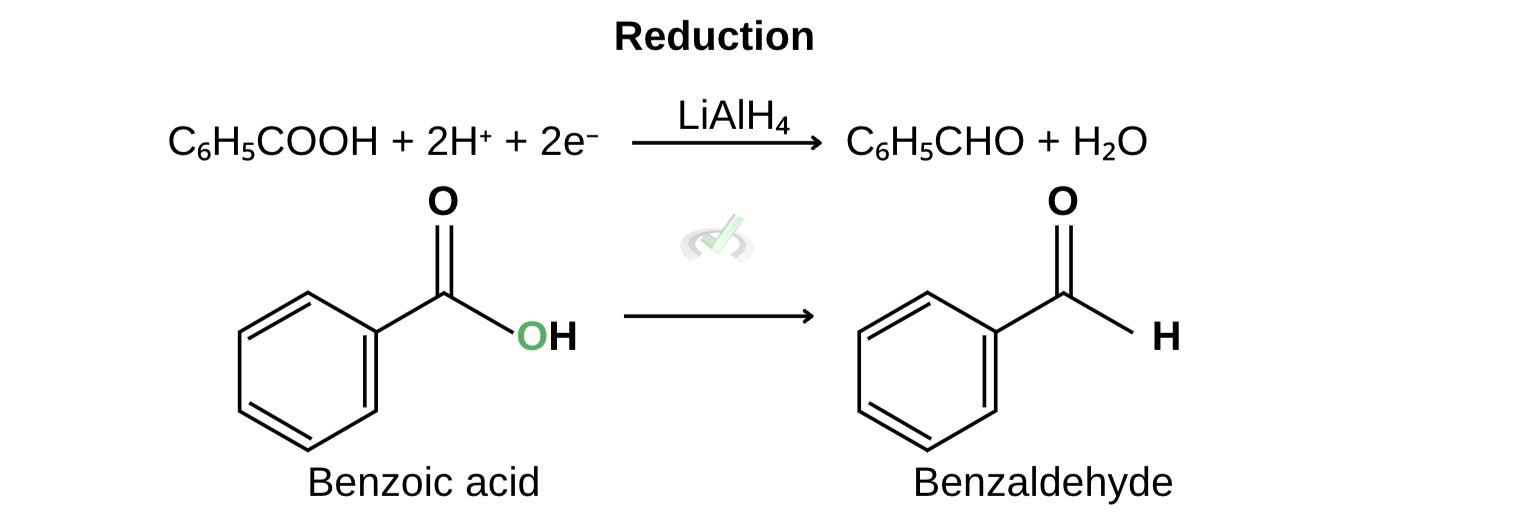
During the reduction of benzoic acid to form benzaldehyde, benzoic acid is reduced and gains 2 electrons and hydrogen to form benzaldehyde.
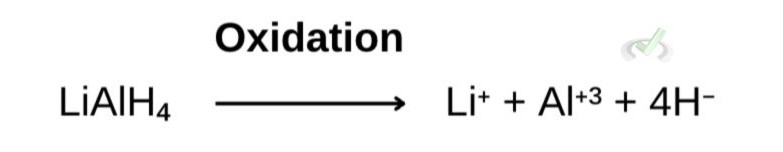
As this happens, lithium aluminum hydride's reducing agent undergoes oxidation and donates or loses electrons or hydride ions (H-).
IV. Conclusion
Redox reactions are important concepts for aldehydes and ketones. Reduction-oxidation reactions go hand-in-hand. When alcohol oxidizes to form an alcohol, it loses electrons; simultaneously, the reducing agent goes through reduction, where it gains electrons. Primary alcohols can go through oxidation twice since they have two hydrogen atoms, which can initiate the loss of electrons. Secondary alcohols, on the other hand, can only go through oxidation once to form a ketone since it only has one hydrogen atom to lose. Unlike primary and secondary alcohols, tertiary alcohols cannot go through oxidation since they do not have hydrogen atoms directly bonded to a carbon atom. We characterize oxidation by the increase in the oxidation state of the carbon attached to the oxygen.
V. Key Terms
- Oxidation - a process wherein a chemical species loses electrons and increases its oxidation state.
- Oxidation State - a number representing the loss or gain of electrons.
- Oxidizing Agent - a chemical species in a redox reaction that initiates oxidation and accepts the electron lost during oxidation.
- Primary Alcohol - an alcohol with a hydroxyl (-OH) group attached to one R group.
- Redox Reactions - reduction-oxidation reactions are a type of reaction that involves the transit of electrons between chemical species.
- Reducing Agent - a chemical species in a redox reaction that initiates reduction by losing electrons.
- Reduction - a process wherein a chemical species gains electrons and decreases its oxidation state.
- Secondary Alcohol - an alcohol with a hydroxyl (-OH) group attached to two R groups.
- Tertiary Alcohol - an alcohol with a hydroxyl (-OH) group attached to three R groups.
VI. Practice Questions
Sample Practice Question 1
What would be the product if we treated benzaldehyde with sodium borohydride?
A. A primary alcohol
B. A secondary alcohol
C. A tertiary alcohol
D. An isopropanol
Ans. A
Sample Practice Question 2
When we treat alcohol with PCC, what role does the alcohol have in the reaction?
A. A weak base
B. A strong base
C. An oxidizing agent
D. A reducing agent
Ans. D







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
