We’ve talked about a lot of reactions so far and most of these, as you may notice, follow the same nucleophilic-substitution pattern. What we know now is that nucleophilic substitution happens through the addition of the nucleophile and elimination of a good leaving group. In this article, we’ll go through two other reactions of carboxylic acids. Specifically, we’ll look at a reaction that involves the breakdown of esters to make soap. We’ll also see one of the chemical reactions used to synthesize various organic compounds.
I. Saponification
Before we get into soap-making, let’s look back at one of the main principles behind saponification.
Hydrolysis is a chemical reaction that involves reacting a compound with water resulting in the breakdown of that compound.
This reaction can occur in two ways: an acid-catalyzed hydrolysis where an ester is broken down into a carboxylic acid and an alcohol using an acid as the catalyst for the reaction. Another way hydrolysis can happen is through base-promoted hydrolysis or saponification.
Saponification happens using a strong base. “Sapo” comes from the Latin word that refers to soap. The term literally translates to making soap!
During saponification, esters break down into soap and alcohol. The breakdown of an ester in a base-catalyzed reaction results in a carboxylate salt (soap) and glycerol (alcohol) as shown in the figure below.
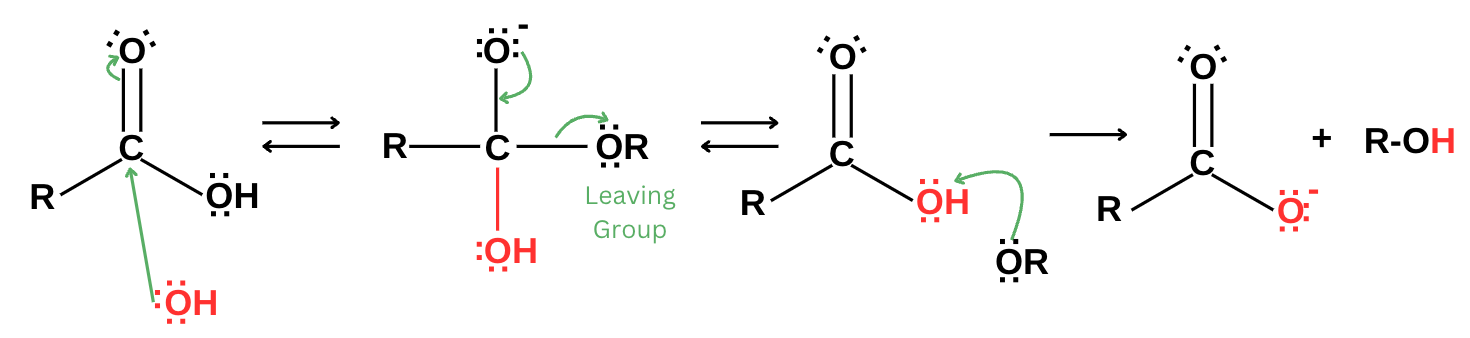
First, a strong base will act as a nucleophile and attack the carbonyl carbon. Further repositioning of electrons will occur to keep the carbon bonded to four atoms only. This will ultimately lead to ejecting a good leaving group. The leaving group, now bearing a negative charge, will protonate to form an alcohol. The final product of the reaction will be carboxylate salt and alcohol.
Example:
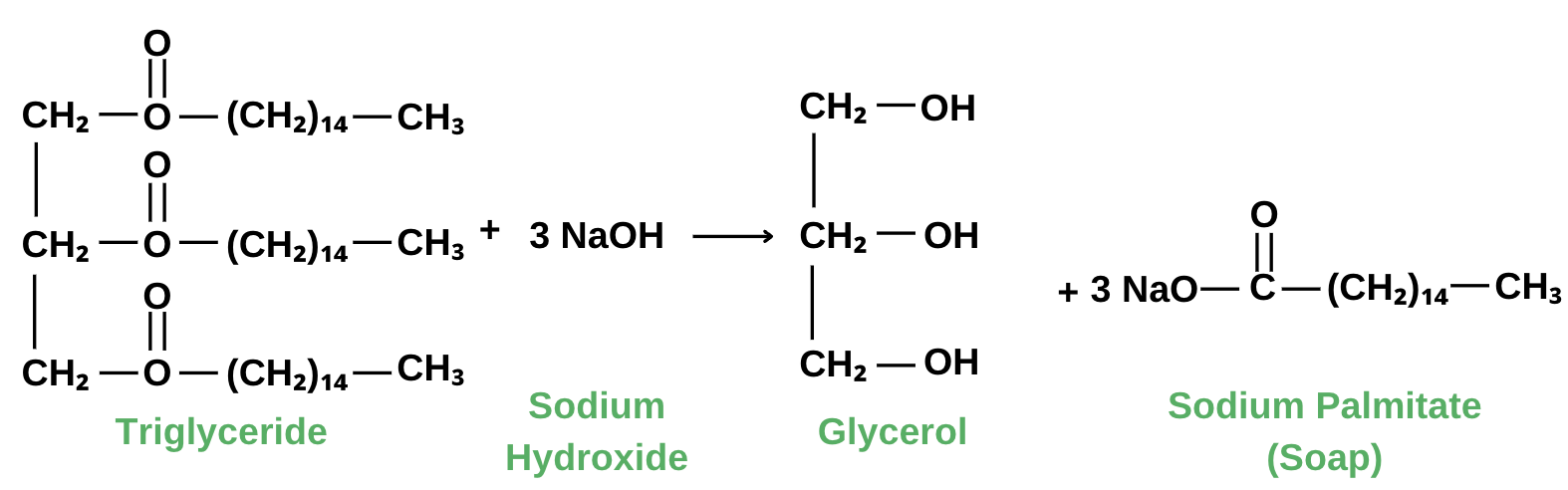
We use fats and a strong base as the main ingredients in soap-making. Triglycerides are usually used in making soap. These compounds are esters of glycerol and fatty acids. When a strong base such as sodium hydroxide (NaOH) is used to react with a fatty acid or oil such as a triglyceride, the reaction yields the formation of alcohol (glycerol) and soap (sodium palmitate).
II. Decarboxylation
When you “de-carboxylate,” you remove a carboxyl group from a molecule. Yes, it’s that simple! Let’s first look at the general formula of decarboxylation.
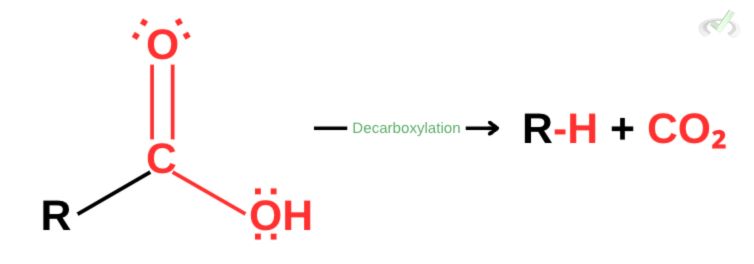
Here, our carboxyl (-COOH) group is removed from the functional group. We also see that carbon dioxide (CO2) is also a product of the reaction. Basically, decarboxylation is a reaction where a carboxylic acid turns into carbon dioxide (CO2).
We see decarboxylation being used in many applications, one of which is by decarboxylation of alpha-ketoglutarate which is an essential element in the Krebs cycle.
Beta-Keto Acids
Compounds with a ketone and carbonyl group attached to adjacent atoms are called -keto acids. The term makes sense since the carbonyl is one carbon away from the carboxyl group, and the ketone is in the beta position.
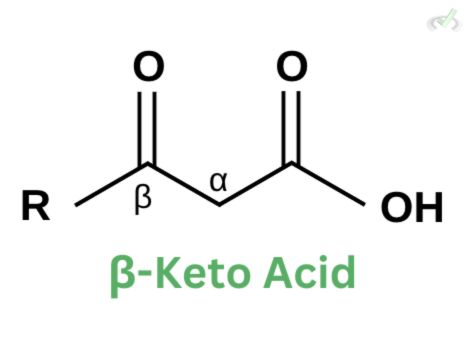
There are two main causes of the ease of decarboxylation:
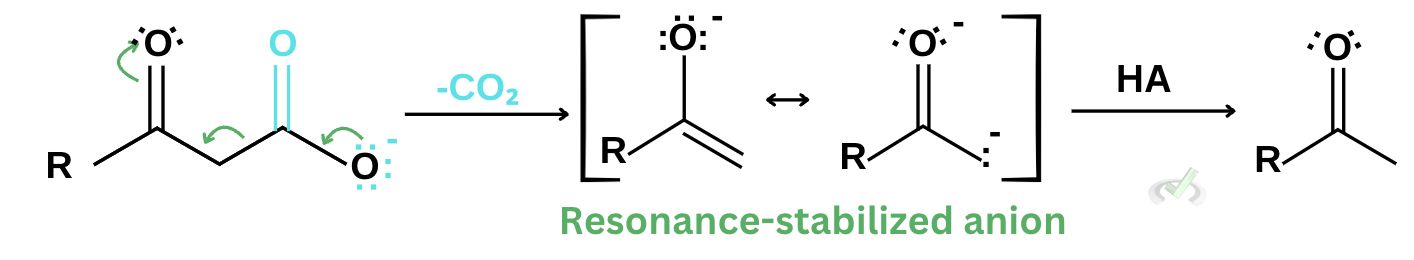
1. During the decarboxylation of an acid, a cyclic transition state allows the rearrangement of the carboxyl group and enolate to facilitate carbon dioxide removal. This provides a more favorable pathway that produces a stable compound such as ketone.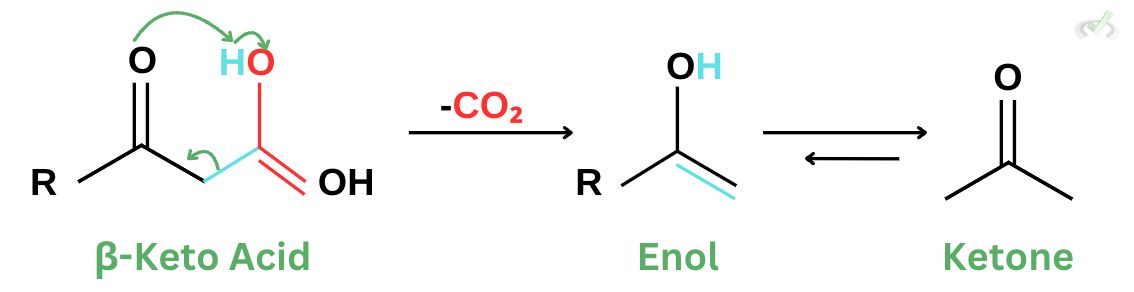
2. Resonance is important in any chemical reaction because it’s another way to provide stability in molecules. Since it allows the rearrangement of electrons without changing the position of the atoms, it minimizes electron repulsion and lowers the overall energy of the structure. Basically, resonance structures allow multiple ways to produce the same product.
For β-keto acids, decarboxylation results in an intermediate resonance-stabilized anion that is much more stable. This anion is also known as an enolate ion.
III. Conclusion
Carboxylic acids undergo a lot of reactions that mainly follow nucleophilic substitution reactions. While the mechanism involves a nucleophile attacking an electrophile and removing a good leaving group, these reactions use different molecules that lead to various derivatives. Saponification is a type of reaction that produces soap and alcohol, this reaction is a base-catalyzed hydrolysis that involves the use of a strong base. Decarboxylation is another reaction that removes the carboxyl group from a molecule in the form of carbon dioxide.
IV. Key Terms
- Decarboxylation - The process of removing a carboxyl group from a parent molecule as carbon dioxide.
- Hydrolysis - A chemical reaction that uses water to break down a compound.
- Resonance - A concept that allows electrons to have different arrangements in a molecule without changing the position of the atoms.
- Saponification - The process of making soap and alcohol via hydrolysis.
V. Practice Questions
Sample Practice Question 1
Which among these compounds cannot undergo decarboxylation?
A)
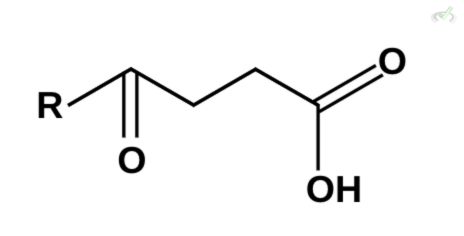
B)
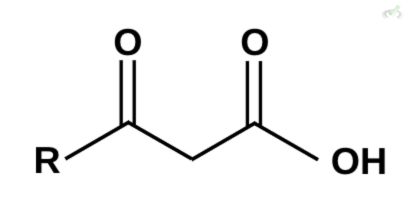
C)
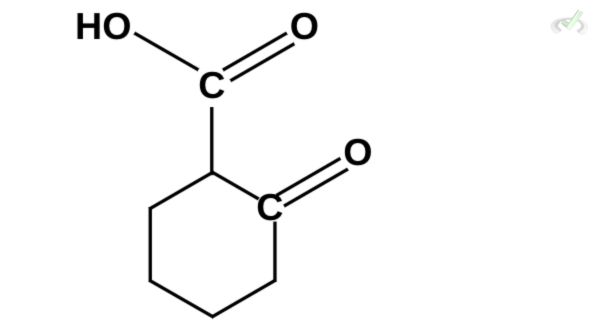
D)
Ans. D
Sample Practice Question 1
Which of these statements is FALSE?
A) Saponification is the breakdown of an ester that produces salt and alcohol.
B) Water is not needed in base-catalyzed hydrolysis.
C) Resonance anions lead to more stable compounds.
D) A beta-keto acid is a carboxylic acid with a carbonyl in the beta-carbon.
Ans. B







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot