I. Polar Molecules and Dipoles
Atoms form covalent bonds through the sharing of electrons. When a covalent bond is present, the compounds formed through these bonds will have a behavior heavily influenced by their valence electrons. Remember that atoms have bonds through their valence electrons. These electrons heavily influence the polarity of a molecule.
Polarity, by definition, is the distribution of an electrical charge across the molecule. Let’s consider hydrogen chloride (HCl) below.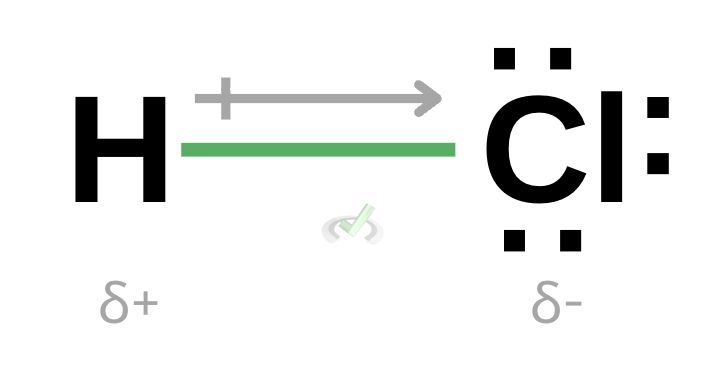
One thing you might notice is the presence of valence electrons on the chlorine atom. These valence electrons show all of the unbonded electrons around the atom. Hydrogen has no free valence electron because its only valence electron is already bonded with one valence electron from chlorine.
The arrow points towards the side with more valence electrons. This arrow represents the negativity of a polar molecule. In this case, there are many electrons on the side of chlorine, so the arrow points towards chlorine. This means that this side is partially negative.
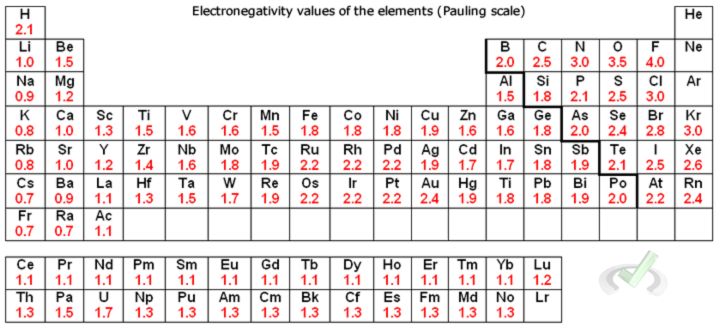
Another concept we can use is electronegativity. This property shows the tendency of an electron to attract and hold on to electrons. If we look at the periodic table of elements and look at the electronegativity values, notice how the electronegativity of chlorine (3.1) is higher than that of hydrogen (2.1). In a polar molecule, the partially negative side has a larger electronegativity.
So what does this mean?
A covalent bond can only be polar or nonpolar. Polar molecules have positive and negative poles, which tells us that one side is more negative and one is more positive. The molecules have poles, this is why they are called polar!
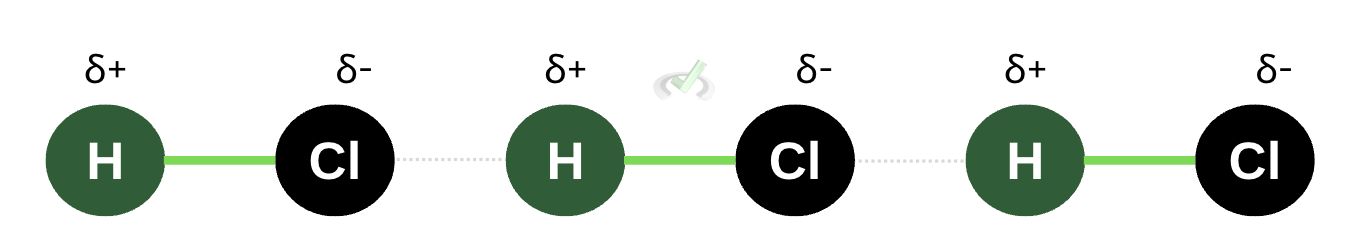
When one side has more electrons than the other, the tendency of electrons or electronegativity is unequal across a polar molecule. This means that it has a dipole moment. A dipole moment indicates an unequal distribution of charges across a molecule. This inequality of charges influences chemical behavior. We see this in dipole interactions where polar molecules tend to be attracted to each other because one molecule’s negative pole is attracted to another’s positive pole. In the image below, we see a dipole interaction among HCl molecules.
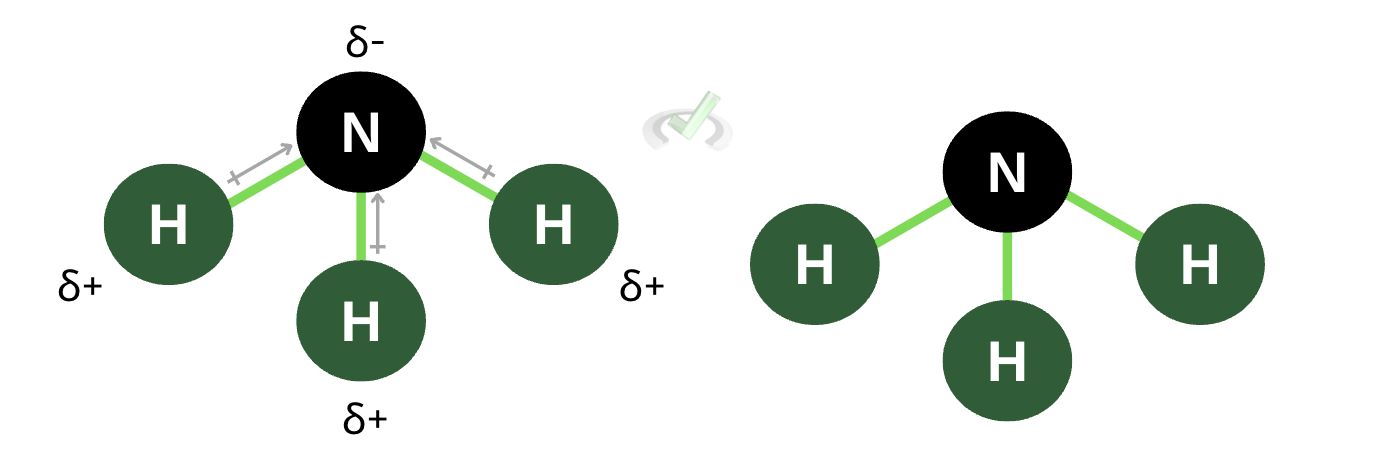
Polarity also influences chemical structure. In molecules with multiple atoms, polarity influences how atoms arrange themselves in a molecule. Since polar molecules have opposite poles, polar molecules tend to pull away from each other. If you notice the structure of ammonia, the Nitrogen atom sticks out, and the hydrogen atoms seem to be pulling away from nitrogen. Aside from being more electronegative, nitrogen has a partially negative charge, which makes the partially positive hydrogen atoms want to pull away from the electrons.
II. Nonpolar Molecules
So, if polar molecules have poles, it makes sense to say that nonpolar molecules do not have poles!
A molecule is nonpolar when it has no dipole moment. To put it simply, nonpolar molecules do not have a positive or negative side. Their charges cancel each other. One such compound is carbon dioxide.
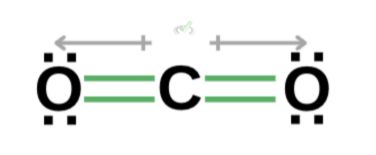
Unlike HCl, which has more electrons on one side than the other, both sides of carbon dioxide are composed of oxygen molecules with the same number of valence electrons. Carbon also fully uses all of its valence electrons to bond with oxygen, balancing the electron distribution across the molecule.
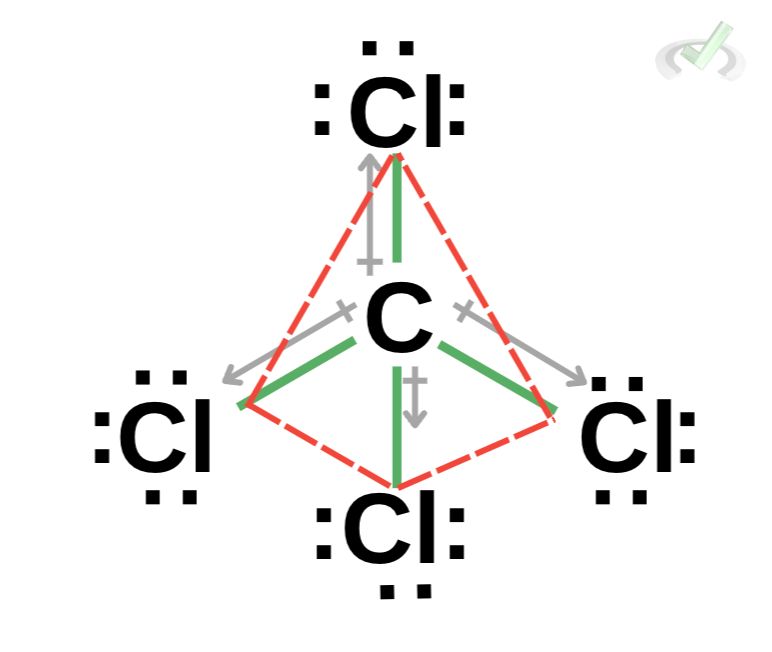
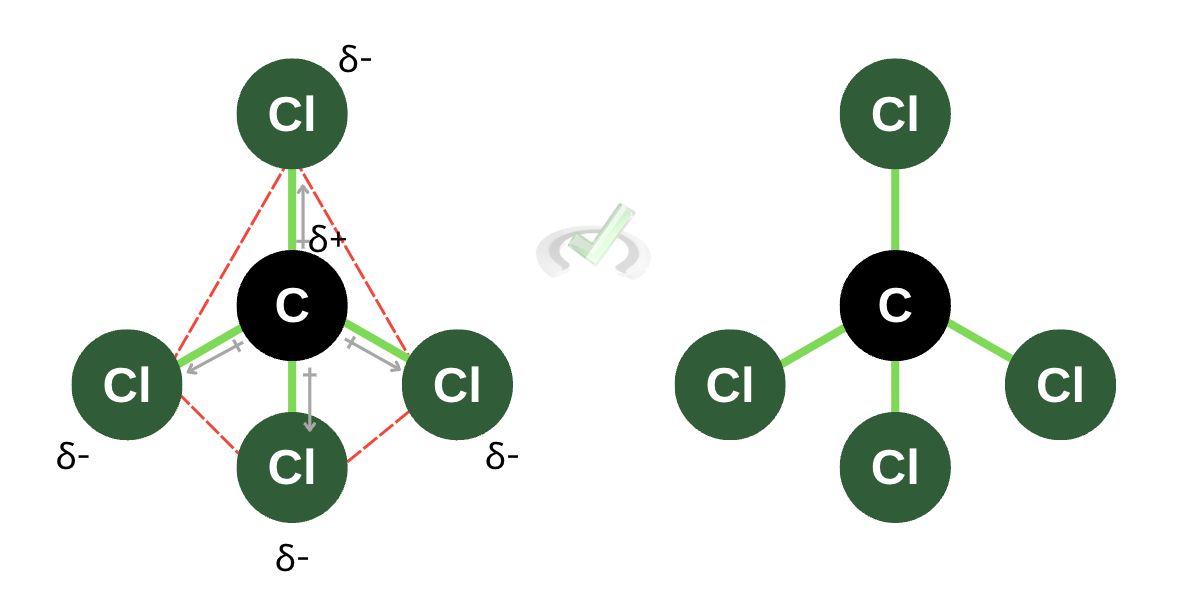
A nonpolar molecule has no dipole moment. It’s a molecule with an equal distribution of charges. The pull of the more electronegative atoms also cancels out a dipole moment. We see this in a carbon tetrachloride (CCl₄) molecule. Because all sides bonded to carbon are all the same species of atoms, they have the same number of valence electrons, and they do not leave any side of the molecule to be more positive than the other.
III. Conclusion
It does seem like a lot to take in, but polarity all comes down to this:
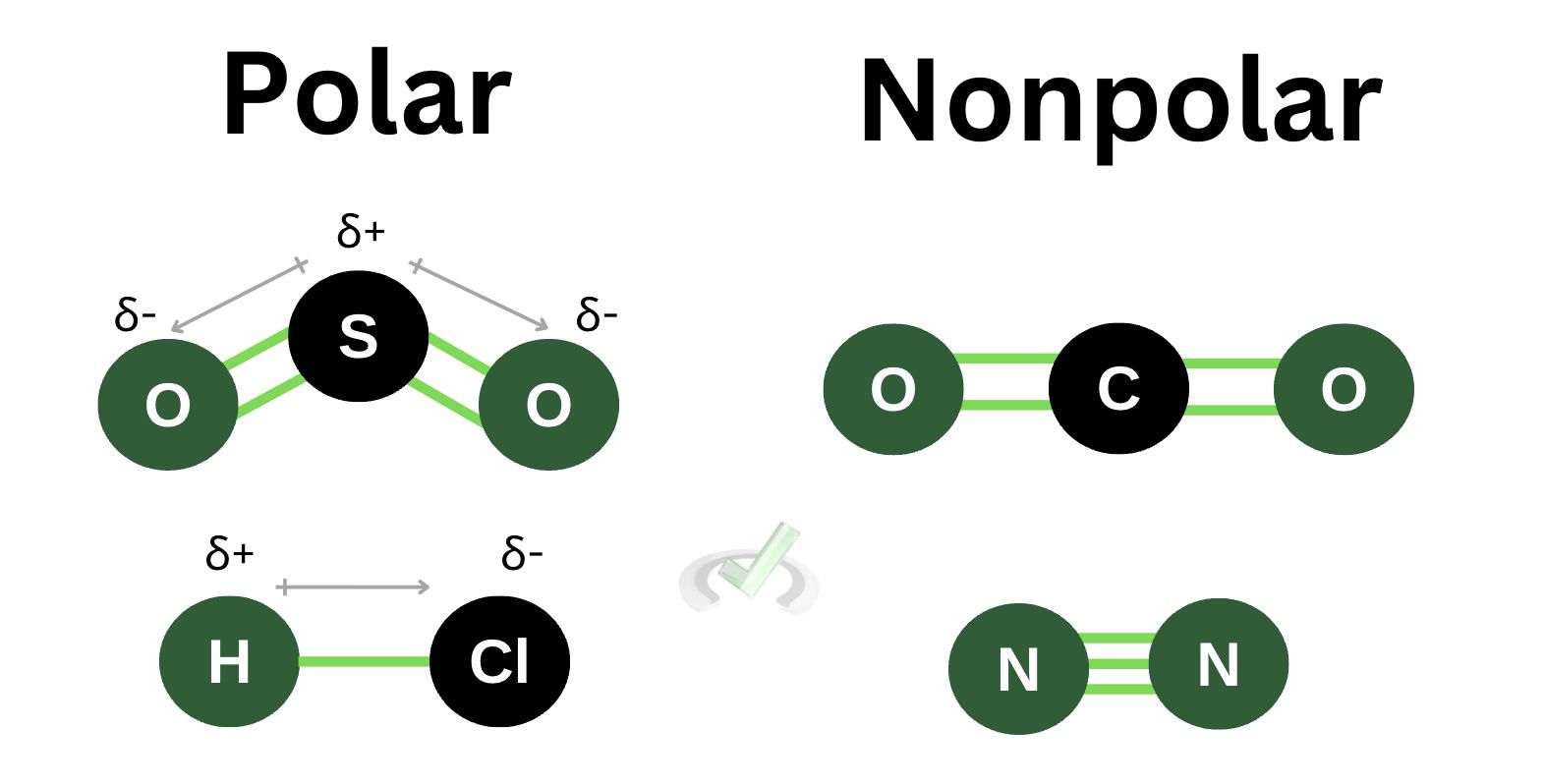
Polar molecules have opposite poles because of their unequal distribution of electrons or charges. The existence of poles creates a dipole moment. All polar molecules have a dipole moment. Nonpolar molecules, on the other hand, have no poles. The charges and distribution of electrons across nonpolar molecules are equal. There is an equal distribution of charges which makes poles impossible. Nonpolar molecules, therefore, have no dipole moment.
IV. Key Terms
- Electronegativity - the tendency of an atom to attract electrons.
- Dipole moment - a measure of the strength of two opposing poles.
- Nonpolar molecule - a molecule with no poles.
- Polar molecule - a molecule with poles.
V. Practice Questions
Sample Practice Question 1
Which of the following is not accurate?
A. All polar molecules have a dipole moment.
B. Polarity exists between molecules with uneven electronegativities.
C. Nonpolar molecules do not have electronegativity.
D. Nonpolar molecules do not have poles
Ans. C
Sample Practice Question 2
The following concepts are true except:
A. Polarity affects the structure of a compound.
B. Opposite poles tend to pull away from each other.
C. The more electronegative atom pulls electrons towards itself.
D. The least electronegative atom pushes electrons away from itself.
Ans. D







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot