The currently accepted atomic model is the electron cloud atomic model. Here, we simply define electrons to be moving around clouds where they are likely to be found. Contrary to older models that single handedly states that an electron revolves around the nucleus in a specific orbit, the current model describes the revolution around the nucleus to be in a region or a cloud where this electron may be.
Niels Bohr, a Danish physicist, gave his own take on how the electrons move around the nucleus and while it does not casually explain that it moves around in a space, his atomic model accurately describes what happens when electrons absorb or release energy. In this article, we’ll talk more about the significance of Bohr’s atomic model and relate it to how we see reactions involving atoms today.
I. Bohr’s atomic model
Bohr proposed that electrons move around the nucleus in circular paths–similar to how planets revolve around the sun in the solar system. He theorized that the distance from the nucleus is related to the energy of the electrons–the farther an electron is, the greater is the energy of that electron. In his atomic model, electrons move around the nucleus. The place where an electron can be found is called a principal shell. He proposed that the shell closest to the nucleus is the most stable shell known as the ground state.
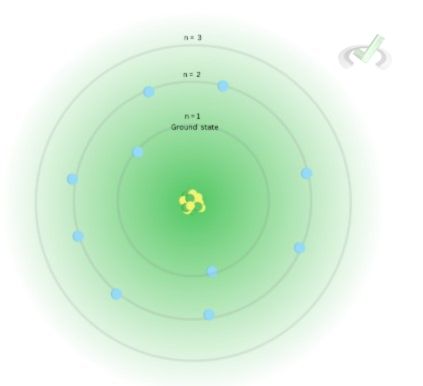
At the ground state, electrons have the least energy. Each shell has a specific energy level, denoted by n (n = 1,2,3, 4). These energy levels correspond to an energy state. This means that electrons at the same energy level have the same energy. Electrons far from the nucleus will have higher energy compared to electrons at energy levels closer to the nucleus.
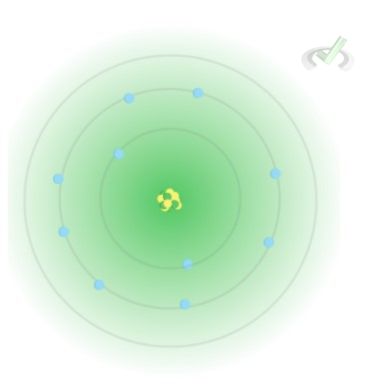
As you may notice, shells can accommodate more electrons as we move up a principal shell. At n=1, we can accommodate 2 electrons; at n=2, the shell can keep up to 8 electrons. We can calculate the number of electrons an electron shell can carry using the formula
Number of electrons =2n²
Where n is the principal shell number.
For the second shell, n = 2. Therefore, the number of electrons that can occupy this space is
= 2(2)² = 8 electrons.
For the third shell where n=3, we get number of electrons=2(3)²=18 electrons.
II. Atomic Spectra
Atomic spectra is the range of energy emitted or absorbed by atoms. Each element has a unique spectrum. This uniqueness is due to the distinct set of energy levels for each element. Spectra is the range of wavelengths or frequencies emitted or absorbed by a substance. This spectra is different for each element.
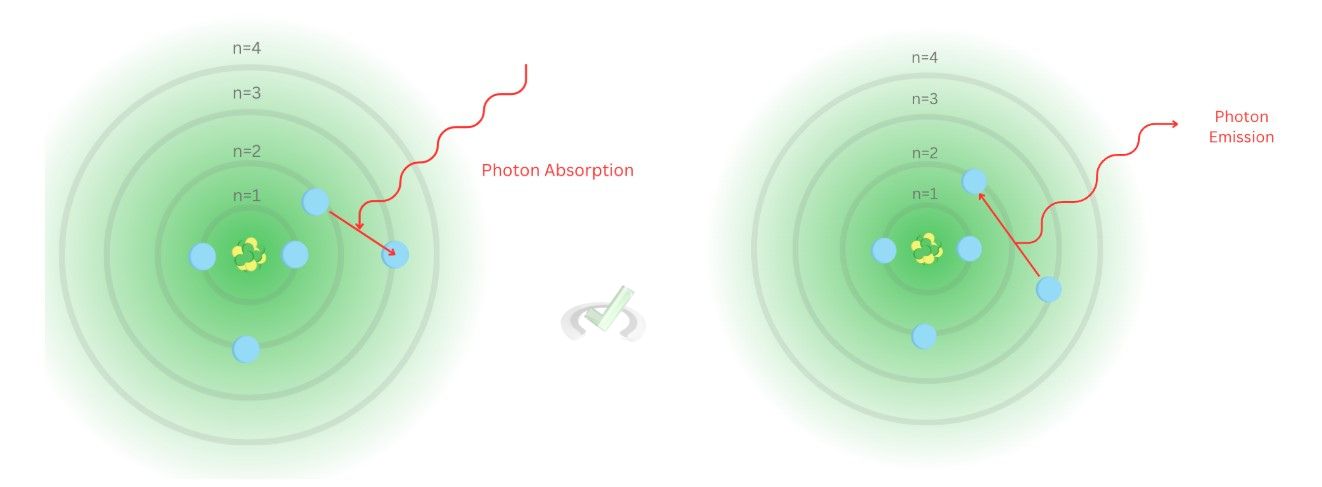
Before we explore these, let’s first make it clear why this concept matters. When an electron absorbs energy, it undergoes excitation. In this state, electrons absorb energy and move to an energy level that can accommodate the energy it receives. This is followed by de-excitation. During de-excitation, electrons release the energy they absorb. This energy can be in the form of heat or light. The color that this de-excitation can reflect depends on the element.
Since light has a wavelength or frequency, the color it reflects should be within the wavelength of energy corresponding to the atomic spectra of the element.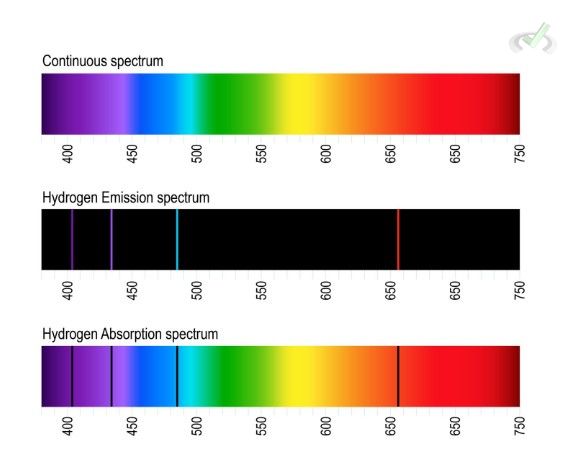
III. Atomic Spectra
The Bohr model highlights one key factor in atomic behavior: the existence of orbitals. These orbitals serve as areas where electrons move around an atom at specific energy levels. Each energy level has an energy corresponding to the amount of energy it can take in. When an electron absorbs energy enough to surpass the energy contained by its shell, it jumps to a higher energy level and undergoes photon absorption. Once it releases the energy it absorbed, it releases the energy back through photon emission. This emission of energy corresponds to a certain wavelength, this wavelength will reflect the color specific to the atomic spectra of the atom. Each element has a different emission spectrum and will reflect different colors of light during de-excitation.
IV. Key Terms
- Atomic Spectra - the spectrum of electromagnetic radiation absorbed or released by an electron during excitation and de-excitation.
- Energy Level -The energy an electron can have in specific orbitals.
- Photon - Energy absorbed or released by an electron.
V. Practice Questions
Consider two electron transitions. In the first case, an electron falls from n = 4 to n = 2, giving off a photon of light with a wavelength equal to 488 nm. In the second transition, an electron moves from n = 3 to n = 4. For this transition, we would expect that:
Sample Practice Question 1
Consider this: In one electron transition, an electron jumps from n = 2 to n = 4. In this transition, we would expect that:
A. Energy is absorbed.
B. Energy is released.
C. Energy is kept at ground state.
D. Energy is non-existent.
Ans. A
Sample Practice Question 2
How many electrons would be accommodated in the fourth shell?
A. 18
B. 32
C. 16
D. 36
Ans. B







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
