We’ve discussed how electrons tend to fill up shells and how each shell corresponds to an energy level that can accommodate a specific energy. We also found that energy levels near the nucleus have the electrons with the lowest energy, and moving up an energy level means that electrons in those energy levels have greater energy.
While energy levels explain the energy of electrons and the process of excitation and de-excitation, this does not give us any information on how electrons are distributed at an energy level or where these electrons might be. We use quantum numbers to better understand the position of electrons and where they’re distributed among shells.
Energy level vs Sublevels
For now, you’ll encounter three terms that might get confusing. These are energy levels, sublevels, and orbitals.
What you have to know about energy levels where electrons might be. To get a general picture of where electrons might be, we first determine their principal quantum number, n. The principal quantum number corresponds to the energy level of an atom. In each shell, we can calculate the maximum number of electrons present using the formula 2n2, where n is the principal quantum number or the energy level.
A subshell is an area within the shell that describes shapes of spaces where electrons might be. Since the atom is three-dimensional, we cannot pinpoint where electrons are in two dimensions. Subshells account for the space that can be occupied by the electrons in three dimensions. Each energy level or shell will have subshells, but the number of subshells will vary per energy level. Orbitals describe the region where electrons are. Each orbital will hold 2 electrons only.
I. Principal Quantum Number
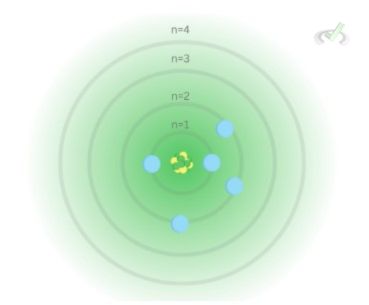
The Principal quantum number describes the shell of an atom. The greater the value for n is, the farther it is from the nucleus. The electron jumps to a higher principal shell during excitation and jumps back to a lower shell during de-excitation. For quantum numbers, principal quantum numbers account for the general area where an electron is.
II. Azimuthal Quantum Number (Angular Momentum)
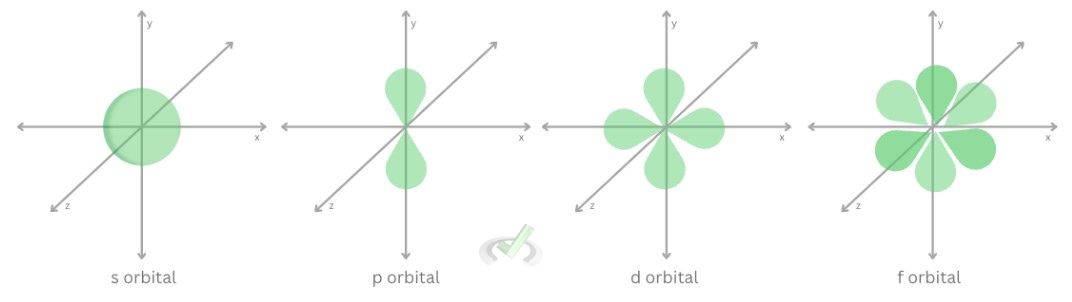
The azimuthal quantum number describes the subshell of an orbital. Azimuthal quantum numbers (l) describe the shape of an electron’s orbital. The values for this can only range from 0 to n-1. These numbers correspond to the orbital shape.
Example: at n = 3, values for l can only be from 0 to 2n | l | subshell |
|---|---|---|
1 | 0 | s |
2 | 0,1 | s, p |
3 | 0, 1, 2 | s, p, d |
4 | 0, 1, 2, 3 | s, p, d, f |
III. Magnetic Quantum Number
Magnetic quantum number describes a more specific description of where an electron might be. It counts for the total number of orbitals, considering the orientation of each orbit. The value for ml ranges from -l to +l.
Subshell | Shapes | 3D shape |
|---|---|---|
s | 1s orbital (1 possible orientation) | 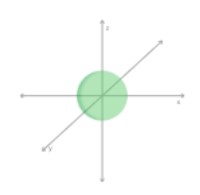 |
p | 3p orbitals (3 possible orientations) | 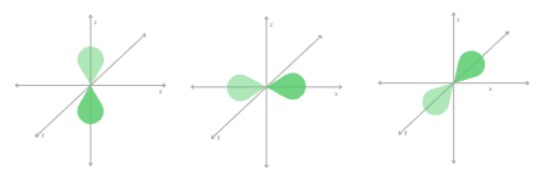 |
d | 5d orbitals (5 possible orientations) | 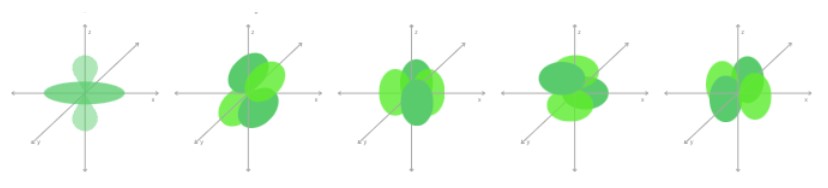 |
f | 7f orbitals (7 possible orientations) | 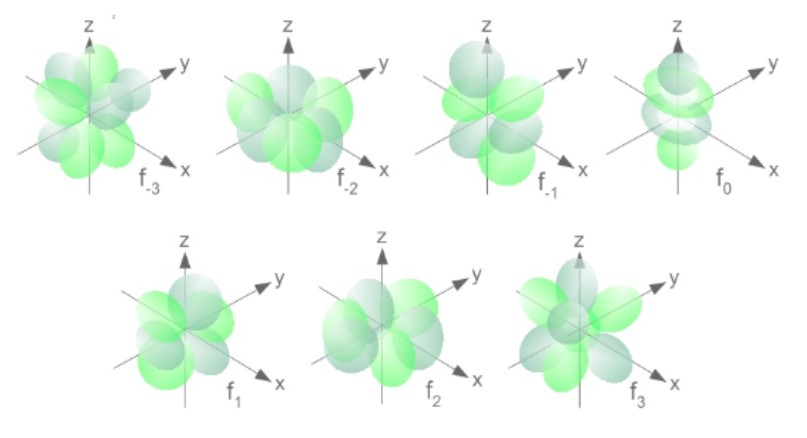 |
IV. Electron Spin Quantum Number
The electron spin quantum number describes the orientation in which the electron is spinning; the possible values can only be +½ for upwards (or clockwise) and -½ downwards (counterclockwide). Let’s briefly discuss the Aufbau principle to understand what a spin is.

Aufbau Principle
This principle lays down four basic things:
- Electrons will fill in the lowest energy level first. This means that for helium that has two electrons, its electrons will be at the 1s orbital since each orbital can take two electrons only.
- The order of orbitals have to be followed with the order being 1s, 2s, 2p, 3s, 3p, 4s, 3d.
- Each electron will hold two electrons with opposite spins. Upwards (clockwise) or downwards (counterclockwise), ½ or -½.
- Electrons will fill up orbitals of the same energy individually first.
V. Other Examples
Let’s try the following examples to understand the concept better.
1. An oxygen atom has eight electrons. Let’s find out the quantum number for the eighth electron.
Principal Quantum Number: Each energy level can take in 2n2 electrons. For n=1, it can take two electrons; for n=2, there can only be a maximum of eight electrons. For oxygen, since there are already 2 electrons at the first energy level, there are 6 electrons left; since the second energy level can take up to eight electrons, all of the 6 electrons can be found at the second energy level. Therefore, n=2.
Azimuthal Quantum Number: The azimuthal quantum number can only take any value from 0 to n-1. Therefore, we can only have 0 and 1 for the value of l.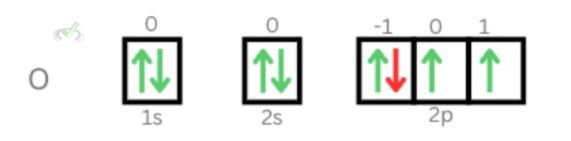
The orbital diagram below shows that the eighth electron can be found at the p orbital. Aufbau’s principle suggests that lower energy levels must be filled in first. Therefore, the eighth electron of oxygen can be found at the p orbital or at l = 1.
Magnetic Quantum Number: This number pinpoints the exact location of where the electron is within the orbital. We also stated that this value will;l range from -l to +l; therefore, the value can only be -1, 0, and 1. Looking at our orbital diagram, we see that the eighth electron is at -1. Therefore, the magnetic quantum number for the eighth electron is mₗ = -1.
Clarifications: Based on the Aufbau principle, each electron will be filled in individually first before pairing it up to fill the same orbitals. The fifth to seventh electron filled in the empty orbitals first. Once all three slots for the orbital are filled in individually, you can pair them up. Since only one electron is left, it fills up at the lowest orbital first.
Electron spin quantum number: Our orbital diagram shows that the eighth electron will be at the 2p orbital. We also see that the electron is spinning downwards. Therefore, the electron spin for the eight electrons is mₛ= -½.
The quantum numbers of the eighth electron of oxygen is: (2, 1, -1, -½)2. Fifth electron of Carbon
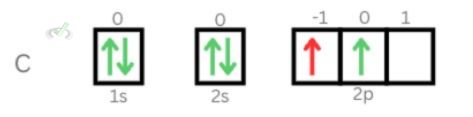
Principal Quantum Number: 2
Azimuthal Quantum Number: 1
Magnetic Quantum Number: -1
Electron Spin Quantum Number: ½
3. Third electron of Neon
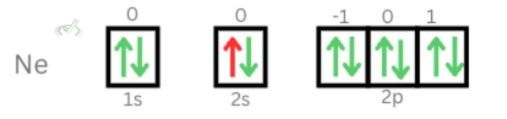
Principal Quantum Number: 2
Azimuthal Quantum Number: 0
Magnetic Quantum Number: 0
Electron Spin Quantum Number: ½
4. Thirteenth electron of Silicon
Principal Quantum Number: 3
Azimuthal Quantum Number: 1
Magnetic Quantum Number: -1
Electron Spin Quantum Number: ½
5. Twentieth electron of Arsenic
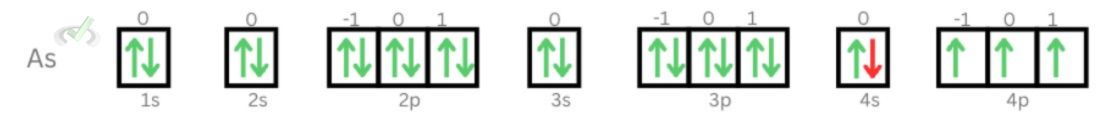
Principal Quantum Number: 4
Azimuthal Quantum Number: 0
Magnetic Quantum Number: 0
Electron Spin Quantum Number: = -1/2
VI. Conclusion
Quantum numbers represent where a specific electron might be and describe its spin. This number can be found in the table below. Using an orbital diagram to figure out how electrons are distributed within an orbital is also helpful. This is prevailed by the Aufbau principle. This principle states that only two electrons can fill in one orbital, electrons in the same orbital should be filled out individually before getting paired up, and electron pairs will have opposite spins–upwards or downwards.
Number | Symbol | Possible Values |
|---|---|---|
Principal Quantum Number | n | 1, 2, 3, 4 |
Azimuthal Quantum Number | l | 0,1, 2, 3, …, (n-1) |
Magnetic Quantum Number | mₗ | -l, …, -1, 0, 1,..., l |
Electron Spin Quantum Number | mₛ | +½, -½ |
VII. Key Terms
Azimuthal Quantum Number - Describes the shape of the orbital; l = 0, s orbital; l = 1, p orbital; l = 2, d orbital; l = 3, f orbital.
Electron Spin Quantum Number - Describes the direction of an electron spin; upwards or clockwise = ½; downwards or counterclockwise = -½.
Magnetic Quantum Number - Describes a more specific space within a sublevel where an electron might be. The value can be from -l to +l.
Principal Quantum Number - Describes the general area or the energy level of where an electron might be.
VIII. Practice Questions
Sample Practice Question 1
Which of the following cannot be a quantum number?
A. (2, 1, 0, 1/2)
B. (1, 0, 0, 1/2)
C. (2, 3, 1, -1/2)
D. (2, 1, -1, 1/2)
Ans. C
Sample Practice Question 2
Determine the quantum number of the second electron of carbon?
A. (1, 0, 0, 1/2)
B. (1, 0, 0, -1/2)
C. (2, 1, 0, 1/2)
D. (2, 0, 0, -½)
Ans. B







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
