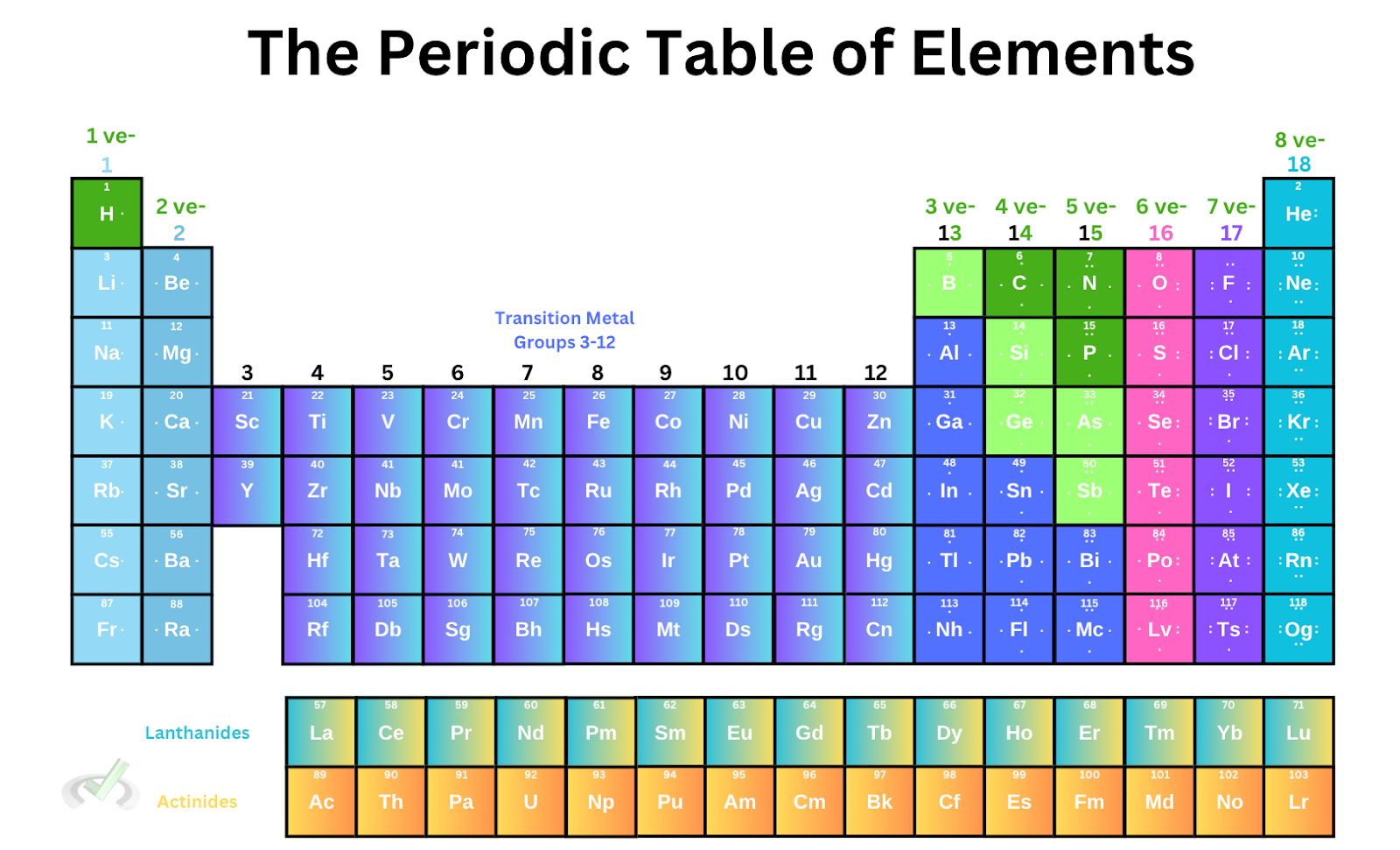
One thing we can observe about the periodic table of elements is that the number of valence electrons is arranged neatly for some groups. The number of valence electrons begins with group 1 having 1 valence electron and group 2 with 2 valence electrons; we skip the transition metals and proceed with groups 13, 14, 15, 16, 17, and 18 with 3, 4, 5, 6, 7, and 8 valence electrons, respectively.
Group 18 in the periodic table has 8 valence electrons (except for He). All elements under group 18 have entirely filled outer shells. We know from our previous articles that noble gasses do not pair with any other element because it has a complete shell. When the valence shell is completely filled, the atom no longer needs to form a bond with other elements since having a complete shell allows stability.
This article will show how elements are written to acknowledge their valence electrons using Lewis dot structures. We’ll also see how these Lewis dot structures help in describing bonds that form between elements. We will also understand one of the fundamental rules in chemical bonding known as the Octet Rule.
I. Lewis Dot Structures
Lewis dot structures show us how many valence electrons are present in an element. We use dots to represent electrons in the outer shell.
Let’s use carbon as an example.
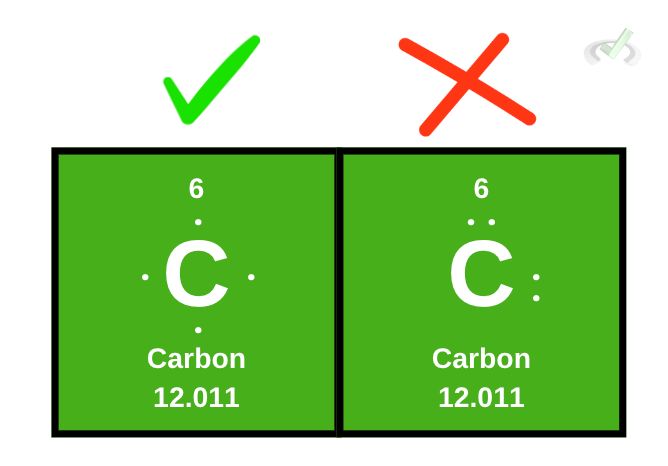
Carbon is found in the 14th group of the periodic table. We can conclude that carbon has four valence electrons. We write Lewis dot structures by individually placing dots around the element. We should not write electrons in pairs if there are still spaces around the element.
When writing the Lewis structures with less than or equal to four valence electrons, there is no specific arrangement to be followed; all that matters is that electrons should fill up the space around the element (left, right, top, and bottom) with at least one electron before being paired up with another.

Here, we see that beryllium’s valence electrons are put on either side of the symbol and not in pairs. For elements with more than four electrons, once all sides of the element are filled with one electron, we can pair it with another electron. Here, we see that nitrogen has 5 valence electrons. Since four electrons will fill up all sides of the symbol, we can put the fifth electron on top to pair with an electron. The same goes for noble gasses. Since argon belongs to group 18, its element is completely surrounded by eight electrons.
By this time, you must wonder why argon is so stable. This is now a good time to discuss the octet rule and how it relates to an atom’s stability.
II. The Octet Rule
Noble gasses are known to be inert. They do not need to form bonds, and they are naturally stable on their own. This stability is due to their completely filled valence shell. When a valence shell is filled with eight electrons, it no longer feels the need to form a bond with any element.
The octet rule explains that atoms tend to form bonds with other atoms to complete their valence electrons. When an atom has less than eight electrons, it’s not at its most stable form. This is why elements bond with each other–it aims to stabilize by sharing or transferring electrons.
Now, let’s see examples to back this up.
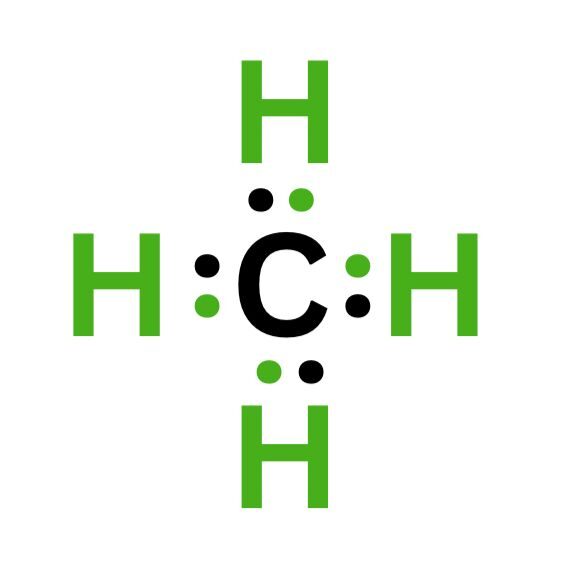
A. Methane

Carbon has 4 valence electrons, and hydrogen has 1 valence electron. Carbon needs four additional electrons to achieve a full valence shell. Therefore, four hydrogen atoms could share their electrons to complete their valence electrons.
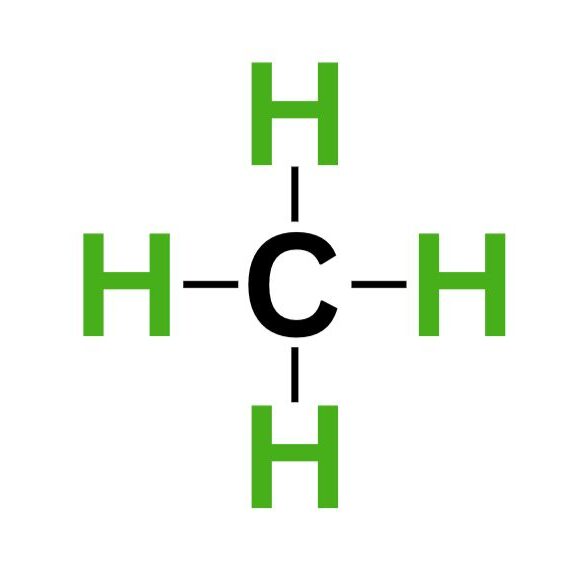
This bond forms CH₄ or methane. We can indicate a bond by writing its Lewis structure using the four dots or straight lines.
Each line connecting hydrogen to carbon represents two electrons. Since there are four lines connecting carbon to each hydrogen atom, carbon now has eight electrons, completing the octet.
B. Carbon dioxide (CO₂)
We know that carbon has 4 valence electrons. For carbon dioxide, we have 2 oxygen atoms. Each oxygen atom will have 6 valence electrons since it belongs under group 16 of the periodic table. Therefore, we have a total of
Now, let’s look at what the bond would look like.

We can first create bonds between carbon and each oxygen atom on either side. Note that carbon is in the middle since it’s the least electronegative atom.

Next, we can complete the octet for each oxygen atom. While looking at this, notice how only the two oxygen atoms have a complete octet. Carbon only has four electrons surrounding it. We can resolve this by moving another pair of electrons to make another bond with carbon.

Since carbon and oxygen share electrons, rearranging their electrons gives both atoms eight electrons surrounding them. Notice that there are now two bonds on each side between carbon and each oxygen atom.
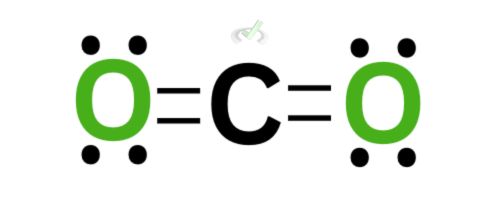
This can be rewritten as a double bond. Since each bond represents two electrons, we can see that carbon has a complete octet by having double bonds on the oxygen atoms. This completes the octet for oxygen as well.
C. Ammonia (NH₃)
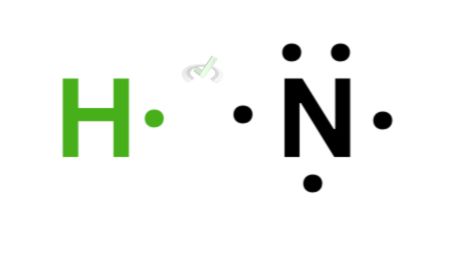
Nitrogen has 5 valence electrons, and it needs three additional electrons to complete its valence shell. Since hydrogen has 1 valence electron, three hydrogen atoms can bond with nitrogen to complete the octet.
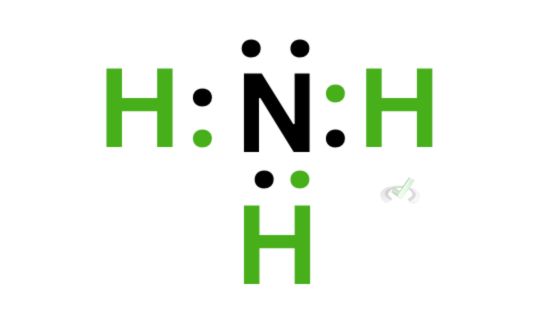
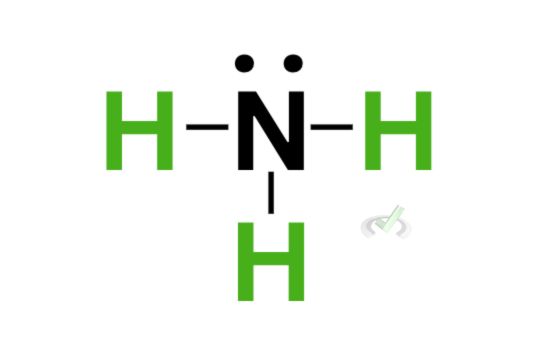
We can then rewrite each bond as a line comprising 2 electrons, which should look like this:
III. Exceptions to the Octet Rule
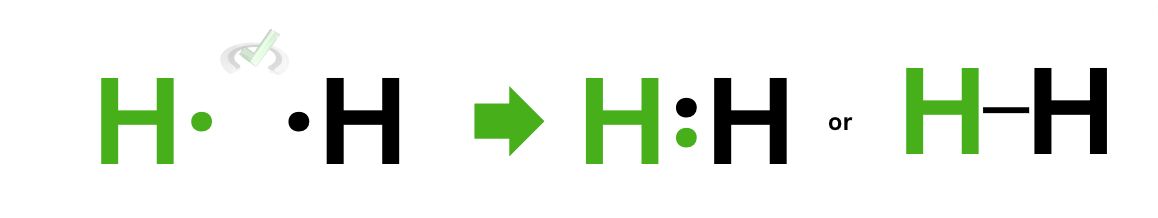
If there’s one thing that’s not as clear for you right now, it’s probably that we keep saying that the octet is complete when hydrogen only has 2 electrons during bonding. This is not a coincidence–the octet rule does not apply to hydrogen or helium because they have their own rule, which is the duet rule.
The duet rule is simple. It only says that an atom (hydrogen or helium) will be stable when it has 2 valence electrons. This is why when hydrogen forms a bond, it does so completely without having to take 7 electrons for itself. The rule also applies to helium because it’s already stable as a noble gas with 2 electrons!
There are also other exceptions to the octet rule. Some molecules are stable with an incomplete octet, and others form bonds with more than eight electrons. These exceptions are not often seen in exams, but it might be helpful to point them out.
IV. Conclusion
Lewis dot structures are a helpful way to visualize elements and their valence electrons. This notation is particularly important when we want to visualize chemical bonding. The structures will only have a maximum of eight electrons since having eight electrons completes the outer shell of an electron. This also explains the octet rule, which states that atoms tend to bond in such a way that they complete their valence electrons by sharing or transferring electrons from other elements.
V. Key Terms
- Lewis dot structures - A form of notation for elements using dots to represent their valence electrons.
- Octet rule - This rule states that atoms tend to form bonds to complete their valence shell with eight electrons.
VI. Practice Questions
Sample Practice Question 1
Which of the following Lewis structures is incorrect?
A.
B.

C.

D.

Ans. D
Sample Practice Question 2
Which of the following molecules is bonded incorrectly?
A.

B.

D.
Ans. D








 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
