Electrochemistry is the branch of chemistry that deals with the relationship between electricity and chemical reactions. A crucial part of electrochemistry is understanding equilibrium in electrochemical systems. This topic combines principles from both chemistry and physics, explaining how reactions can generate electrical energy and how electrical energy can drive chemical reactions.
I. Introduction to Electrochemical Equilibrium
Electrochemical equilibrium occurs when the forward reaction (oxidation) rate equals the backward reaction (reduction) rate. It's when the oxidation and reduction processes happen at the same rate, and no net change is observed in the concentrations of reactants and products. This balance is crucial because it determines the stability and efficiency of electrochemical cells.
Oxidation
- Oxidation is the process where a substance loses electrons.
- Example: In the oxidation of zinc in an electrochemical cell, zinc metal (Zn) loses two electrons to form zinc ions (Zn²⁺).
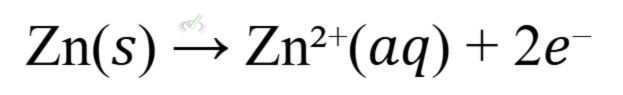
Reduction
- Reduction is the process where a substance gains electrons.
- Example: In reducing copper ions in an electrochemical cell, copper ions (Cu²⁺) gain two electrons to form copper metal (Cu).
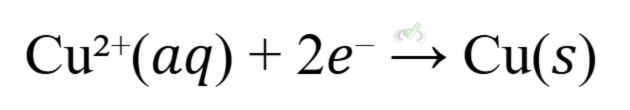
II. Electrochemical Cells
Electrochemical cells convert chemical energy into electrical energy or vice versa. There are two main types: galvanic (or voltaic) cells and electrolytic cells.
A. Galvanic Cells
- Galvanic Cells generate electrical energy from spontaneous redox reactions.
- These cells consist of two metals connected by a salt bridge or a porous disk.
- Example: The Daniell cell consists of a zinc electrode in zinc sulfate solution and a copper electrode in copper sulfate solution.
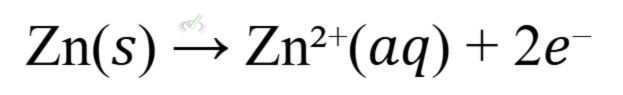
(oxidation)
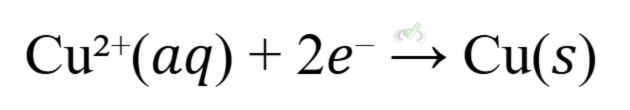
(reduction)
B. Electrolytic Cells
- Electrolytic Cells use electrical energy to drive non-spontaneous reactions.
- These cells are used in processes like electroplating and electrolysis.
- Example: Electrolysis of water, where an electric current splits water into hydrogen and oxygen gases.
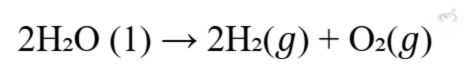
III. Nernst Equation
The Nernst equation relates the cell potential to the concentrations of the reactants and products. It's crucial for calculating the cell potential under non-standard conditions.
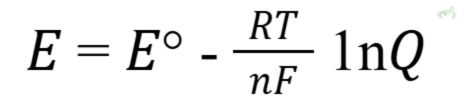
Where:
- E is the cell potential.
- E0 is the standard cell potential.
- R is the gas constant (8.314 J/(mol·K)).
- T is the temperature in Kelvin.
- n is the number of moles of electrons transferred.
- F is the Faraday constant (96485 C/mol).
- Q is the reaction quotient.
IV. Standard Electrode Potentials
Standard electrode potentials (E°) are measured under standard conditions (1M concentration, 1 atm pressure, 25°C). They indicate the tendency of a half-cell to be reduced, and these values are used to calculate the overall cell potential.
Example:
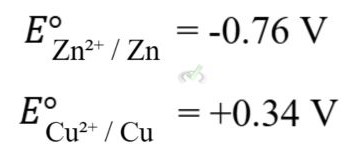
V. Electrochemical Series
The electrochemical series ranks elements by their standard electrode potentials. This helps predict the direction of redox reactions. Metals at the top (like lithium) are good reducing agents, while those at the bottom (like fluorine) are good oxidizing agents.
VI. Concentration Cells
Concentration cells generate electrical energy from the concentration difference of the same substance in two half-cells. The Nernst equation is used to calculate the potential of these cells.
Example:
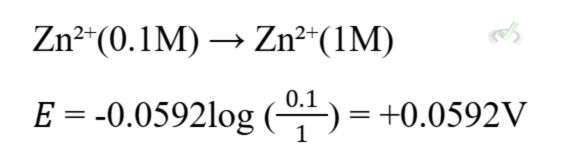
VII. Applications of Electrochemical Equilibrium
Electrochemical principles are used in various real-world applications, from batteries to corrosion prevention.
A. Batteries
- Batteries are galvanic cells that store chemical energy and convert it to electrical energy.
- Example: Lithium-ion batteries, commonly used in smartphones and laptops.
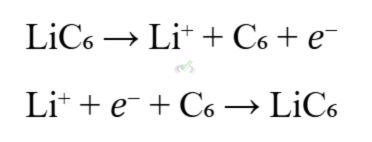
B. Corrosion
- Corrosion is an undesirable electrochemical reaction where metals are oxidized, leading to deterioration.
- Example: Rusting of iron.
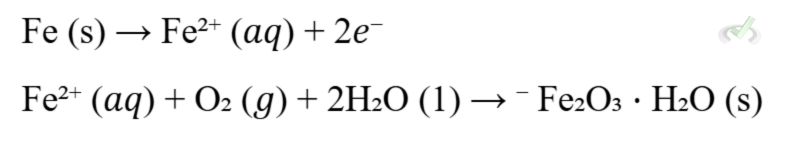
VIII. Bridge/Overlap
Understanding electrochemical equilibrium provides a foundation for exploring broader topics in chemistry and beyond.
A. Relationship with Thermodynamics
Thermodynamics: Electrochemical cells and equilibrium concepts are closely tied to thermodynamics, involving enthalpy, entropy, and Gibbs free energy.
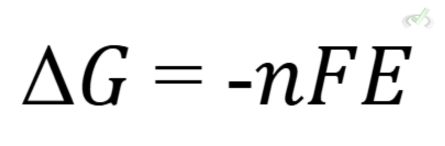
ΔG is the Gibbs free energy change, and E is the cell potential.
B. Impact on Renewable Energy
Renewable Energy: Electrochemical cells are pivotal in renewable energy technologies, such as fuel cells and solar cells, which help produce sustainable energy.
(in a hydrogen fuel cell)
C. Biological Systems
Biological Systems: Redox reactions are fundamental in biological processes, such as cellular respiration and photosynthesis.
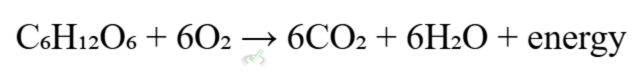
(cellular respiration)
IX. Wrap-Up and Key Terms
Understanding equilibrium in electrochemistry is essential for many scientific and industrial applications. Mastering these concepts helps predict and control chemical reactions that involve the transfer of electrons.
Key Terms
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
- Electrochemical Cell: Device converting chemical energy to electrical energy (or vice versa).
- Galvanic Cell: Generates electrical energy from spontaneous reactions.
- Electrolytic Cell: Uses electrical energy to drive non-spontaneous reactions.
- Nernst Equation: Relates cell potential to concentrations of reactants/products.
- Standard Electrode Potentials: Measure of a half-cell's tendency to be reduced.
- Electrochemical Series: Ranking of elements by their electrode potentials.
- Concentration Cell: Generates electricity from concentration differences.
- Gibbs Free Energy: ΔG=−nFE
X. Practice Questions
Sample Practice Question 1
What happens to the cell potential if the concentration of Zn²⁺ increases in a zinc-copper galvanic cell?
A. It increases.
B. It decreases.
C. It stays the same.
D. It fluctuates.
Ans. B
When looking at the structures of aspartate & lysine, it can be seen that aspartate does have an extra carboxyl group while lysine does have an extra lysine group.
This should make sense, as in physiological pH, the carboxyl group is deprotonated and takes on a negative charge (-1) while the amino group is still protonated and takes on a positive charge (+1).
Hence, this is why aspartate is a negatively charged amino acid while lysine is a positively charged amino acid.
Sample Practice Question 2
Which of the following is an example of a reduction reaction?
A. Zn (s) → Zn²⁺ (aq) + 2e⁻
B. Cu²⁺ (aq) + 2e⁻ → Cu (s)
C. 2H₂O (l) → 2H₂ (g) + O₂ (g)
D. Fe²⁺ (aq) → Fe³⁺ (aq) + e⁻
Ans. B
When looking at the structures of aspartate & lysine, it can be seen that aspartate does have an extra carboxyl group while lysine does have an extra lysine group.
This should make sense, as in physiological pH, the carboxyl group is deprotonated and takes on a negative charge (-1) while the amino group is still protonated and takes on a positive charge (+1).
Hence, this is why aspartate is a negatively charged amino acid while lysine is a positively charged amino acid.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
