Electrochemical cells convert chemical energy into electrical energy or vice versa. These cells are fundamental in many technologies, including batteries and electroplating. In this article, we'll explore the different types of electrochemical cells, how they work, and their applications.
I. Introduction to Electrochemical Cells
Electrochemical cells are divided into two main types: galvanic (voltaic) and electrolytic. Let's start by understanding the basics of each.
Galvanic Cells
Galvanic cells convert chemical energy into electrical energy through spontaneous redox reactions, in which oxidation and reduction occur naturally.
Oxidation: The process of losing electrons.
Reduction: The process of gaining electrons.
For example, in a simple galvanic cell made of zinc and copper:
(oxidation at the anode)
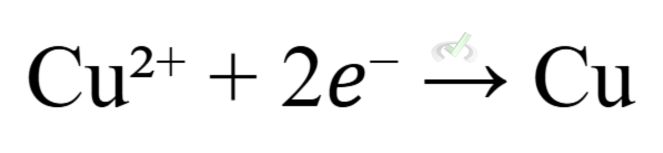
(reduction at the cathode)
In this reaction, zinc (Zn) loses electrons to form zinc ions (Zn²⁺), which are oxidized. Copper ions (Cu²⁺) gain electrons to form copper metal (Cu), which is reduced.
Electrolytic Cells
Electrolytic cells use electrical energy to drive non-spontaneous chemical reactions. They require an external power source to force the redox reaction.
For instance, in the electrolysis of water:
During this process, water (H₂O) is split into hydrogen (H₂) and oxygen (O₂) gases. At the anode, water is oxidized to form oxygen gas and hydrogen ions:
At the cathode, hydrogen ions gain electrons to form hydrogen gas:
II. Detailed Look at Galvanic Cells
Structure and Function
A typical galvanic cell consists of two electrodes: an anode (where oxidation occurs) and a cathode (where reduction occurs). These electrodes are immersed in electrolyte solutions, and a salt bridge connects the two solutions to maintain charge balance.
Example: Daniell Cell
The Daniell cell is a classic example of a galvanic cell. It involves a zinc anode and a copper cathode:
(oxidation at the zinc anode)
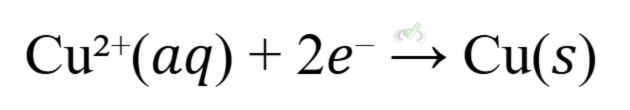
(reduction at the copper anode)
This cell generates an electric current as electrons flow from the zinc to the copper through an external circuit.
Applications
- Batteries: Many batteries, such as the alkaline battery, are based on galvanic cell principles.
- Corrosion Prevention: Galvanic cells help understand and prevent corrosion by sacrificial anodes. These anodes are metals that are intentionally oxidized to protect the main metal.
III. Detailed Look at Electrolytic Cells
Structure and Function
Electrolytic cells have an anode and cathode but use an external power source to drive the reaction. The direction of electron flow is opposite to that of galvanic cells.
Example: Electroplating
Electroplating uses electrolytic cells to coat a metal object with a thin layer of another metal. For example, silver plating involves:
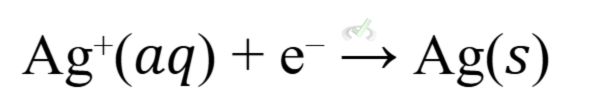
In this process, the object to be plated is made the cathode. Silver ions in the solution gain electrons (are reduced) to form a thin layer of silver on the object's surface.
Applications
- Metal Refining: Electrolytic cells purify metals such as copper. Impure copper is used as the anode, and pure copper is deposited at the cathode.
- Water Splitting: Electrolysis of water produces hydrogen and oxygen gases. This reaction is essential for producing hydrogen fuel.
IV. Comparison Between Galvanic and Electrolytic Cells
Feature | Galvanic Cell | Electrolytic Cell |
|---|---|---|
Energy Conversion | Chemical to Electrical | Electrical to Chemical |
Reaction Type | Spontaneous | Non-Spontaneous |
Anode | Negative (oxidation) | Positive (oxidation) |
Cathode | Positive (reduction) | Negative (reduction) |
Example | Batteries, corrosion cells | Electroplating, metal refining |
V. Bridge/Overlap
Understanding electrochemical cells connects to many areas in chemistry:
Thermodynamics
Studying energy changes in electrochemical reactions helps understand reactions' spontaneity and energy requirements. Gibbs free energy (ΔG) and the cell potential (E°) are critical concepts in this area.
Kinetics
Reaction rates in electrochemical cells are crucial for battery efficiency and electroplating quality. Factors like temperature, concentration, and surface area affect these rates.
Environmental Chemistry
Electrochemical cells play a role in pollution control, such as using electrolytic cells for waste treatment. For example, electrocoagulation is used to remove contaminants from water.
Biological Systems
Electrochemical principles are key to understanding processes like cellular respiration and photosynthesis. In cellular respiration, glucose is oxidized to produce energy (ATP), while in photosynthesis, water is split to produce oxygen.
VI. Wrap-Up and Key Terms
Understanding electrochemical cells involves grasping several key concepts and terms. These cells are crucial in both theoretical and practical chemistry applications. To wrap up, electrochemical cells are essential in many technologies, from batteries to metal refining. They help us understand fundamental chemical reactions and their practical uses.
Key Terms
- Anode: Electrode where oxidation occurs.
- Cathode: Electrode where reduction occurs.
- Electrolyte: Solution that conducts ions.
- Salt Bridge: Device that maintains charge balance in galvanic cells.
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
- Gibbs Free Energy (ΔG): Energy associated with a chemical reaction that can do work.
- Cell Potential (E°): Measure an electrochemical cell's voltage.
VII. Practice Questions
Sample Practice Question 1
In a galvanic cell, which electrode is the site of oxidation?
A. Cathode
B. Anode
C. Salt Bridge
D. Electrolyte
Ans. B
In a galvanic (or voltaic) cell, oxidation occurs at the anode, where electrons are lost from oxidizing substances. These electrons then flow through the external circuit to the cathode, where reduction occurs. The mnemonic "An Ox, Red Cat" can help us remember that the Anode is the site of Oxidation, and Reduction occurs at the Cathode.
Sample Practice Question 2
What type of reaction occurs in an electrolytic cell?
A. Spontaneous
B. Non-Spontaneous
C. Both A and B
D. Neither A nor B
Ans. B
In an electrolytic cell, a non-spontaneous reaction occurs. This means the reaction requires an external power source, as it does not happen independently under standard conditions. The electrical energy supplied drives the chemical reaction, the opposite of what happens in a galvanic cell, where a spontaneous reaction generates electrical energy.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
