At first glance, the term “thermochemistry” may come off as a little daunting and scary in terms of its content matter; however, as with other topics on the MCAT, breaking down the terms one by one makes things a lot easier!
Taking the term piece by piece, the prefix “-thermo” simply refers to anything relating to heat. Likewise, the suffix “chemistry” should be self-explanatory: put together, thermochemistry is simply the chemistry of heat, specifically heat transfer.
This chapter overview will cover all the foundational topics related to thermochemistry. Let’s dive in and get started!
Thermochemistry on the MCAT: What You Need to Know
Topics on general chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
Try to expect around 2-4 questions that can appear on the MCAT covering stoichiometric topics, probably with a higher emphasis on calculation based questions!
Introductory general chemistry accounts for 30% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Thermochemistry
The great thing about thermochemistry that you can use to your advantage is that there’s significant overlap with our “Thermodynamics on the MCAT!” as given in our MCAT Physics module!
It’s definitely beneficial — and something that we encourage — for you all to pair studying these chapter overviews and their corresponding articles together within a close time frame in order to maximize the overlap and understanding!
1. Important Thermodynamic Terminology, Processes, and Functions
Before getting into the more complex topics of thermochemistry, it’s first important to establish some of the basic fundamental terminologies in order to understand the more complicated thermodynamic content.
As thermochemistry deals with the study of heat transfer, we have specific terms to assign to the components involved in the transfer of heat. The 2 important terms to know are the system and the surroundings.
The system refers to the portion of the universe that we’re interested in studying. Conversely, the surroundings refer to the rest of the universe, not including the system. Put together, we can study how heat is transferred between the system and surroundings.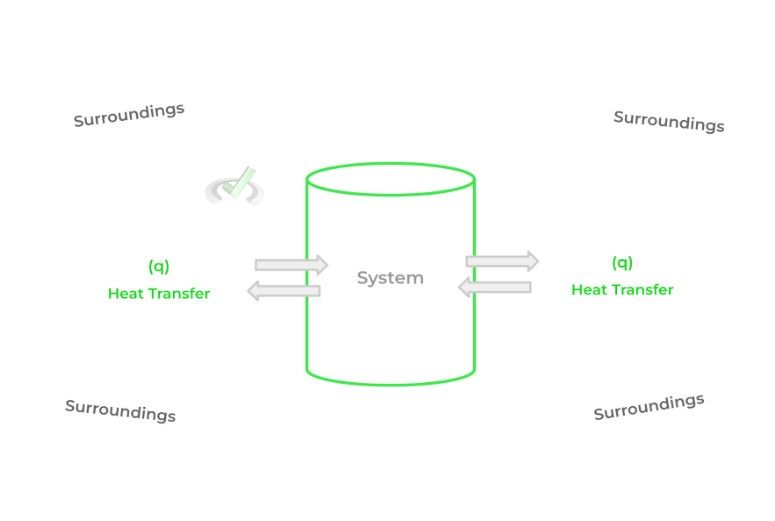
The difficult part about applying thermodynamics to chemistry is identifying and creating the boundaries of what’s considered the system and what’s considered the surroundings!
In the case of thermochemistry, we’re interested in how the heat released from chemical reactions is transferred — this is a great introduction when we talk about calorimetry and Hess’ law in the upcoming sections!
Additionally, another important topic to understand is thermodynamic functions, which are basically mathematical values that can be used to describe the changes in the equilibrium state!
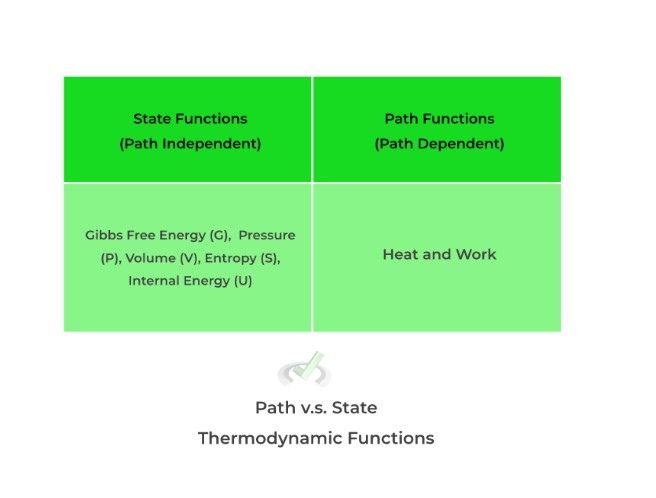
Thermodynamic functions (better thought of as thermodynamic “states”) can be categorized into path/process or state/point functions: path/process functions DEPEND on the path/course it takes to from one equilibrium state to another while state/point DO NOT depend on the path/course.
Take a look at some examples below! Right now, don’t focus too much on the technicalities and rationales (we’ll cover those in the in depth articles) — for now, just focus on internalizing these to memory.
Finally, we can describe changes in the thermodynamic equilibrium states utilizing thermodynamic processes: these are essentially processes that describe how the thermodynamic equilibrium of a system changes.
This best applies to the first law of thermodynamics which gives an equation for how the internal energy of a system changes. Sometimes when there is a change in equilibrium, we can hold a certain value constant such as volume, temperature, etc.!
When one of these values is held constant, we can reduce and simplify the equation found in the first law of thermodynamics! In addition, these thermodynamic processes where we hold one value constant have specific names as shown below:
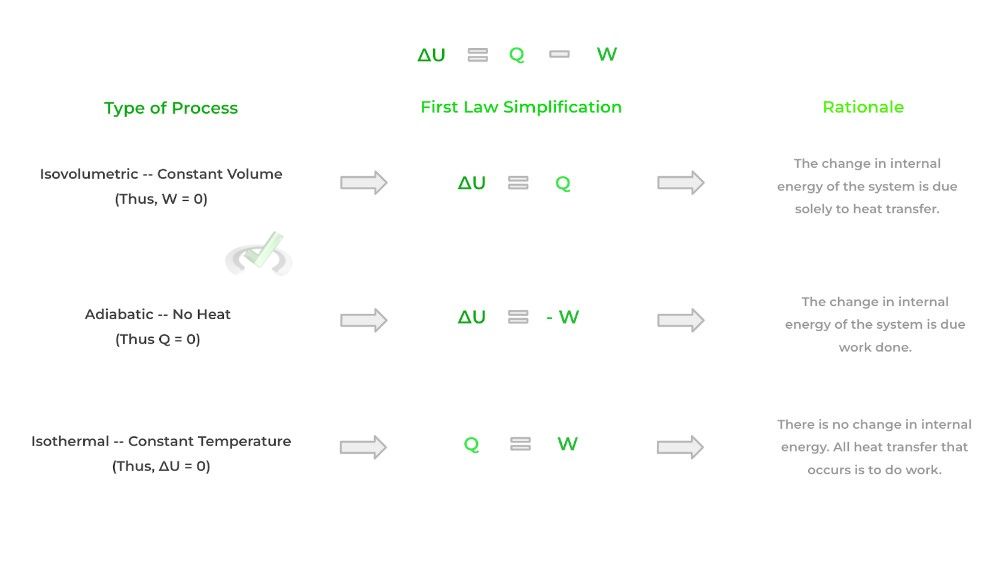
To sum it up, thermodynamic functions describe the mathematical quantities of the equilibrium state of a system while thermodynamic processes describe how we get to the change in equilibrium state!
Full Study Notes : Important Thermodynamic Terminology, Processes and Functions
For more in-depth content review on the differences between the terminology that describes thermodynamics, check out these detailed lesson notes created by top MCAT scorers.
2. Heat v.s. Enthalpy
A topic that can be a little tricky to navigate around is understanding the difference between heat and enthalpy, as these are 2 related terms in thermodynamics. Let’s first differentiate between the 2 terms before getting into more complex topics!
Heat (usually designated as “q”) refers to the transfer of thermal energy between bodies with different temperatures. Thermal energy refers to the total kinetic energy of a system as a result of all the vibrating particles.
When 2 bodies are placed in contact with one another, thermal energy will be transferred — in the form of heat — from the higher energy body to the lower energy body, eventually resulting in an equilibrium!
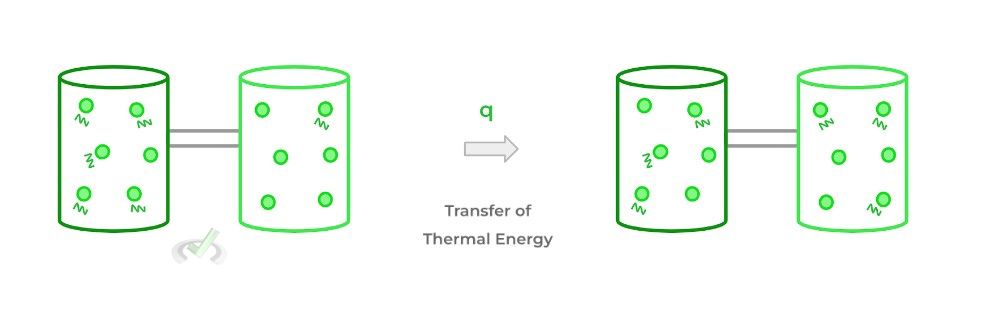
Enthalpy, on the other hand, can really be thought of as the energy change of a system due to heat transfer! Specifically, in order for this to be true, the change in energy and heat transfer must occur at constant pressure.
For the scope of the MCAT, this is a great way to think about enthalpy! Remember: enthalpy is nothing more than a way to keep track of energy changes of a system via heat transfer at constant pressure.

Full Study Notes : Heat v.s. Enthalpy
For more in-depth content review on the differences between heat and enthalpy, check out these detailed lesson notes created by top MCAT scorers.
3. Thermodynamics of Phase Changes
Now that we covered heat and enthalpy, we can go on to discuss how these two are involved in how a substance changes from different states of matter: solid, liquid, and gasses.
Just as a quick refresher to basic science: a solid is a state of matter with definite shape and volume, a liquid is a state of matter with a definite volume but not shape, and a gas is a state of matter with no definite volume or shape.
Another important aspect to consider when discussing the differences between the states of matter is the strength of the intermolecular interactions: on the extreme end, solids have the strongest intermolecular interactions while gasses have the weakest as shown by the visual below: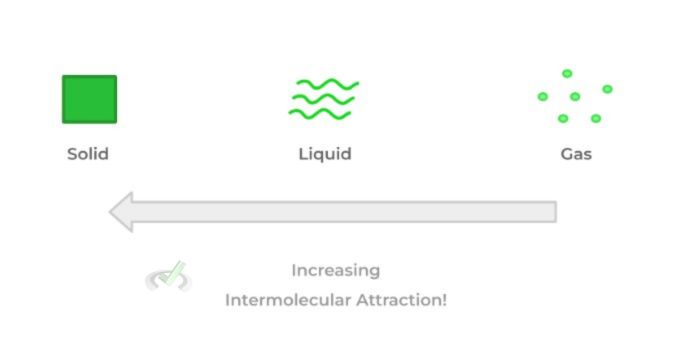
Furthermore, we also must differentiate between 2 important terms dealing with enthalpy: exothermic and endothermic. These terms are used to describe chemical reactions where there’s an exchange of energy in the form of heat.
An exothermic reaction occurs when a reaction releases energy in the form of heat and has a negative value; conversely, an endothermic reaction absorbs energy in the form of heat and has a positive value — let’s actually model this via a reaction diagram!
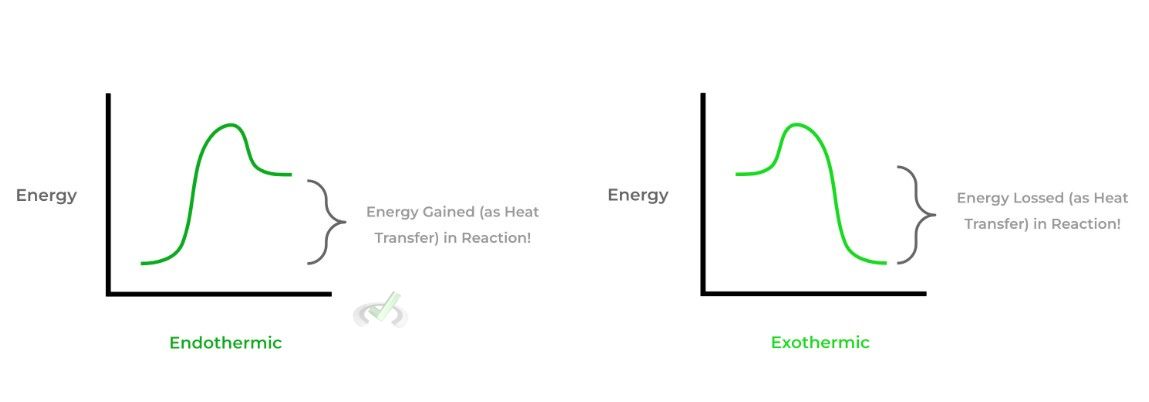
Now having gone over these two terms, perhaps you guys can make an educated guess on what type of reaction is occurring when a solid is melting into a liquid (and vice versa) — after a few, take a look below to see if your guess was right!

It should make sense from our talk about intermolecular interactions why going from a solid to liquid to gas phase is endothermic: energy is required in order to overcome the intermolecular attractive forces!
When put together, the changing state of matter for a substance can be displayed in what’s called a phase change diagram. This diagram models the change of a substance’s state of matter, plotting temperature and time as shown below:
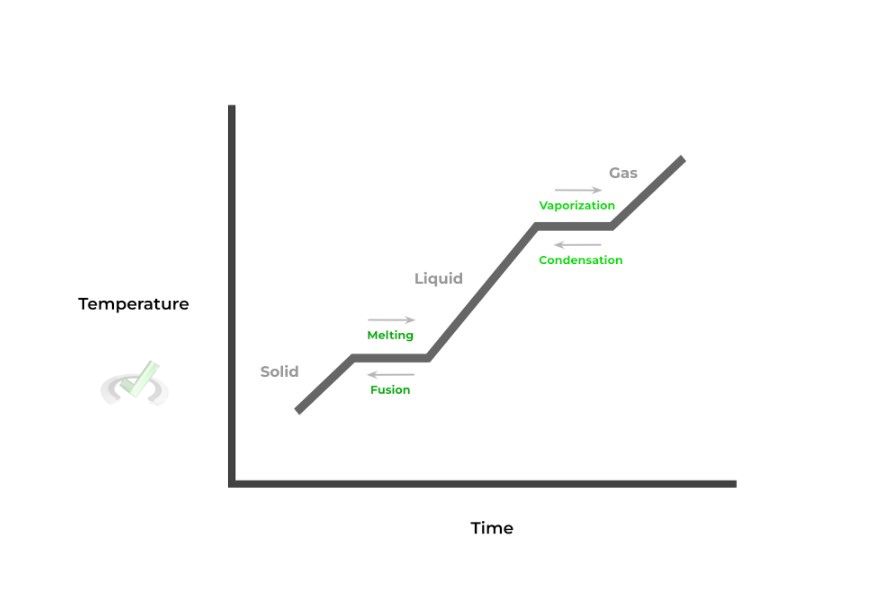
Shown above is a typical phase diagram with all the corresponding name changes from one state to another (i.e. solid to liquid → melting).
Full Study Notes : Thermodynamics of Phase Changes
For more in-depth content review on thermodynamics of phase changes, check out these detailed lesson notes created by top MCAT scorers.
4. Calculations with Heat and Enthalpy
There are 3 main types of calculations to be familiar with come test day: calorimetry calculations, Hess’ law, and bond dissociation energy calculations — we’ll give you a brief overview for these calculations and go much more in depth in our other articles!
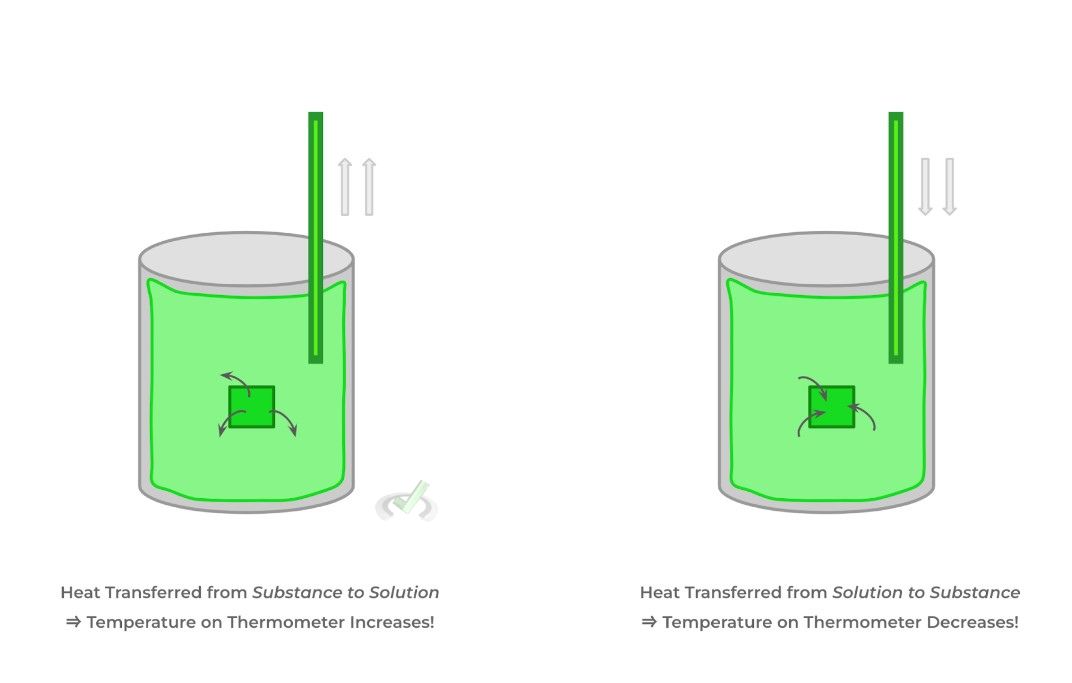
Calorimetry refers to a technique where we can measure the heat transfer between 2 objects: oftentimes, this is done by placing a substance in a solution (usually water) and measuring how the temperature of the solution changes.
From here, we can make a couple of important relationship to measure the heat transfer:
I. If the temperature of the solution increased (i.e. ΔT = +), heat was transferred from the substance to the solution.
II. If the temperature of the solution decreased (i.e. ΔT = -), heat was transferred from the solution to the substance.
An important equation to become comfortable with is the specific heat capacity equation which gives the amount of heat that a substance must absorb (or release) in order to change the temperature of that substance, as shown below:

We’ll get more into how to use this equation in our article, but the important variable to note for now is the specific heat capacity, (Cp). This term is unique for each substance (although differs for different states of matter) and describes the amount of energy needed to raise the temperature of 1 gram of substance by 1˚C.
Hess’ Law is also an important calculation to know for the MCAT, which basically allows us to calculate the change in enthalpy of a reaction! Specifically, we can use the fact that simpler reactions and their enthalpic values are additive!
As shown above, in order to get the enthalpy value for the bottom chemical reaction, the 2 reactions above are added together as well as their enthalpic values in order to summate to the full chemical reaction as well as its reaction enthalpy.
Finally, we can also find the enthalpy of a reaction utilizing the bond dissociation energies. These are the enthalpic values which show the amount of energy required in order to break a chemical bond. You’ll often find these values listed in a table below:
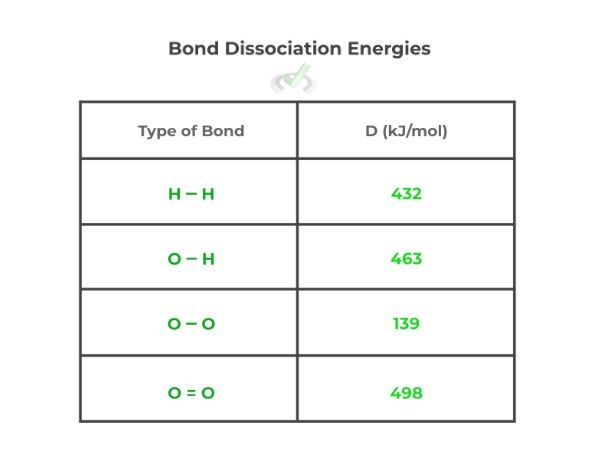
In order to find the enthalpy of a reaction, we can summate the amount of energy it takes to break the reactants’ bonds and the energy released when the products’ bonds are formed. This can be given by the equation below:

This is just a brief introduction to some of the calculations you’ll need to know in regards to enthalpy and heat.
Full Study Notes : Calculations with Heat and Enthalpy
For more in-depth content review on calculations with heat and enthalpy, check out these detailed lesson notes created by top MCAT scorers.
5. Entropy in Chemical Reactions
The concept of entropy is defined by the 2nd law of thermodynamics which states that the entropy (S), also commonly rephrased as the disorder, of the universe is always increasing — here, the universe includes both the system and the surroundings.
Although associated with disorder, entropy is better thought of as the tendency for energy to be dispersed when permitted to do so. Consider for example the case of gas particles trapped in a container; when a hole is punctured in the container, the gas particles flow out spontaneously which increases the entropy!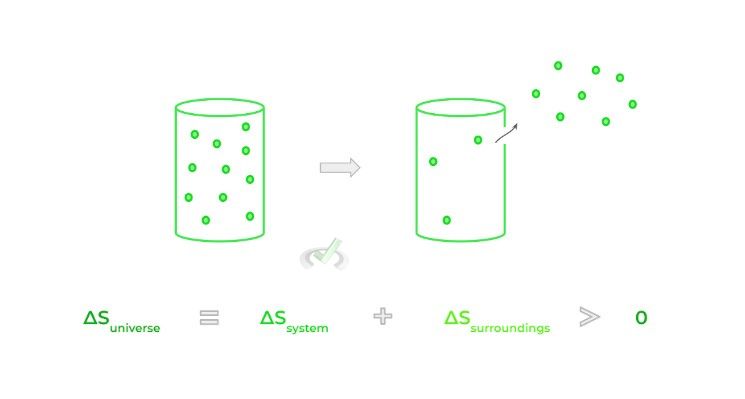
This now explains why thermal energy is transferred from a hot substance to a cold substance in the form of heat: when put in contact with one another, the transfer of energy is permitted and allows for a spontaneous dispersal of thermal energy, generating thermal equilibrium.
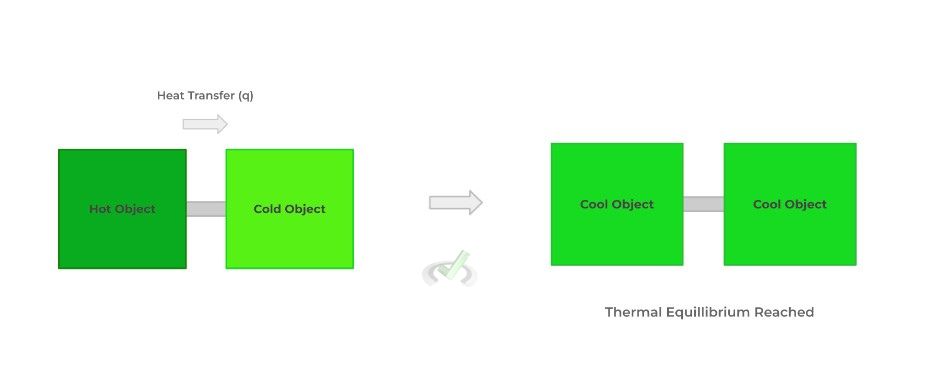
As you’ll see coming up, understanding entropy we’ll be important for when we cover Gibbs free energy in the next section!
Full Study Notes : Entropy in Chemical Reactions
For more in-depth content review on entropy in chemical reactions, check out these detailed lesson notes created by top MCAT scorers.
6. Gibbs Free Energy and the Spontaneity of Chemical Reactions
Finally, a key concept that always comes around in the context of chemistry, especially biochemistry, is the Gibbs free energy. Specifically, we’re focused on the change in Gibbs free energy of a reaction which takes into account how the enthalpy and the entropy of a reaction change upon completion of a reaction.
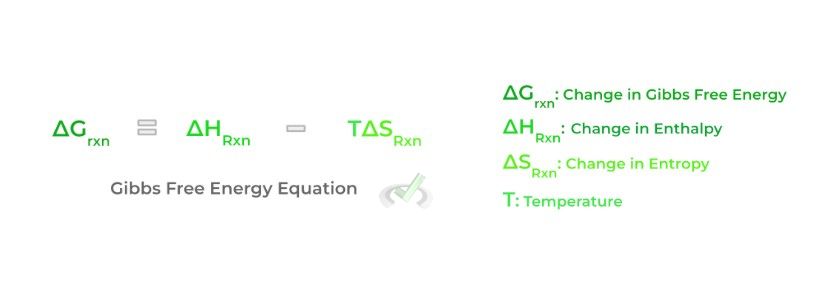
The change in Gibbs free energy of a reaction details the spontaneity of a reaction: this simply refers to how likely a reaction will progress without the need for external energy input — from this, we’ll introduce some important terms and relationships involved with Gibbs free energy:
I. If ΔG is negative, the reaction is spontaneous. It is also termed exergonic as energy is released in the reaction and the products are lower in energy than the reactants.
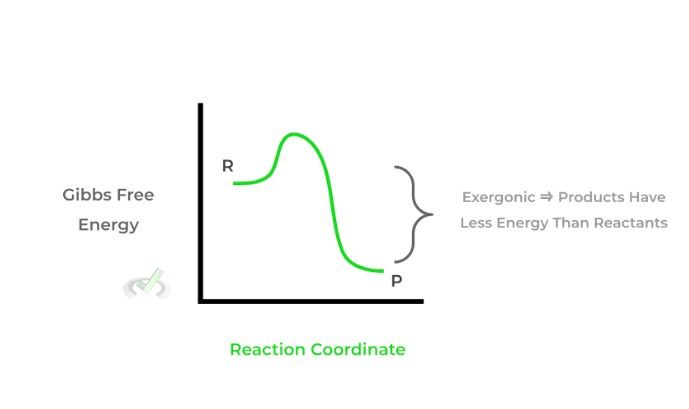
II. If ΔG is positive, the reaction is nonspontaneous. It is also termed endergonic as energy is gained in the reaction and the products are higher in energy than the reactants.
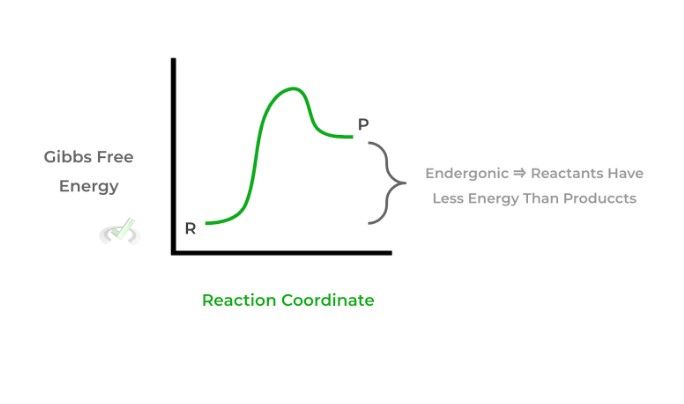
Understanding the changes in Gibbs free energy for a reaction and whether they’re spontaneous or nonspontaneous can give a lot of information about a reaction — give this article a read for more information!
Full Study Notes : Gibbs Free Energy and Spontaneity of Chemical Reactions
For more in-depth content review on Gibbs Free Energy and spontaneity of chemical reactions, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about thermochemistry in general chemistry!
Term | Definition |
|---|---|
System | The portion of the universe we’re interested in studying |
Surrounding | Includes all the other portions of the universe except the system |
Path/Process Function | A type of thermodynamic function which DOES depend on the path taken to get from one equilibrium state to another |
State/Point Function | A type of thermodynamic function which DOES NOT depend on the path taken to get from one equilibrium state to another |
Thermodynamic Process | Describes the path taken to get from one thermodynamic equilibrium state to another |
Heat |
Refers to the transfer of thermal energy from one body to another |
Enthalpy |
A thermodynamic quantity which attributes the change in the energy state of a system to the loss (or gain) of thermal energy via heat transfer — in order for the enthalpic value to equal the value of heat, constant pressure must be established |
Solid |
State of matter which has definite volume and shape |
Liquid |
State of matter which has definite volume but not definite shape |
Gas |
State of matter which has no definite volume and no definite shape |
Exothermic |
Describes negative enthalpy value where a reaction loses energy in the form of heat |
Endothermic |
Describes a positive enthalpy value where a reaction gains energy in the form of heat |
Calorimetry |
A technique which can determine heat transfer between a substance and a solution; the substance is submerged in a solution (usu. water) and a thermometer measures the change in temperature of the solution |
Specific Heat Capacity |
A number unique to a substance and refers to the amount of energy needed in order to raise the temperature of 1 gram of a substance by 1 C˚ |
Hess’ Law |
States that the enthalpy values simpler chemical reactions can be added in order to summate to an overall reaction enthalpy value |
Entropy |
Thermodynamic value which describes the “disorder” of a system; Usually better thought of as the tendency for energy to disperse when permitted to |
Gibbs Free Energy |
A value (specifically the change in Gibbs free energy) which describes the spontaneity of a reaction |
Exergonic |
Term to describe the release of Gibbs free energy in a reaction; Often used in synergy with spontaneous |
Endergonic |
Term to describe the gain of Gibbs free energy in a reaction; Often used in synergy with nonspontaneous |
Additional FAQs - Thermochemistry On the MCAT
Is Thermochemistry on the MCAT?
Is Thermodynamics on the MCAT?
Why is Thermodynamics So Hard? – MCAT
What is Hess’ Law? – MCAT
Additional Reading Links – Study Notes for Thermochemistry on the MCAT
Additional Reading: General Chemistry MCAT Topics:
- Atomic Structure on the MCAT
- Periodic Table on the MCAT
- Bonding and Chemical Reactions on the MCAT
- Chemical Kinetics on the MCAT
- Electrochemistry on the MCAT
- Equilibrium on the MCAT
- Solutions on the MCAT
- Stoichiometry on the MCAT
- The Gas Phase on the MCAT
- Acids and Bases on the MCAT
- Redox Reactions General Chemistry MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
