When we add an acid or base to an aldehyde or ketone, we initiate a reaction that forms an enol. We briefly discussed how this happens in our previous article on aldehydes and ketones.
A little side note: the “keto” form we’re talking about in this process is the carbonyl group that’s attached to a functional group. Therefore, “keto” refers to both aldehydes and ketones!
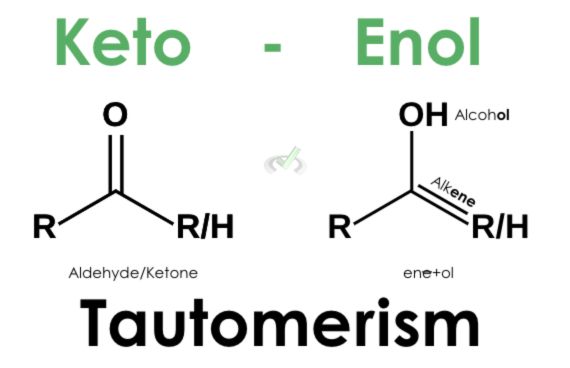
We also figured that enolates form when hydrogen from an alpha carbon is deprotonated by a strong base. In this process, since the enolate ion is unstable, the electron pair will collapse to form a carbon-carbon double bond.
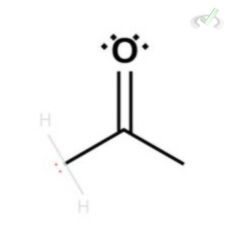
However, this forces one bond from the carbonyl oxygen to also collapse since carbon can only hold four bonds. This leaves oxygen with a negative charge and three lone pairs of electrons. This instability due to the charge is resolved by the protonation of the oxygen using free hydrogen atoms around the solution. What we end up having is an enol which is an alcohol with a double bond.
Just so we could breathe a little better, let’s take this process into bite-sized chunks.
I. Keto-Enol Tautomerization in Parts
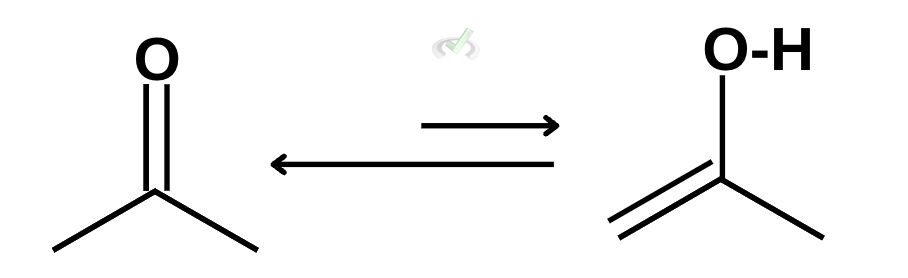
During tautomerization, we see how ketones can transform into an enol once it’s catalyzed by an acidic or basic solution. Let’s take this ketone for example:
To transform this into an enol, we want to remove the double bond from the carbonyl atom and place it elsewhere.
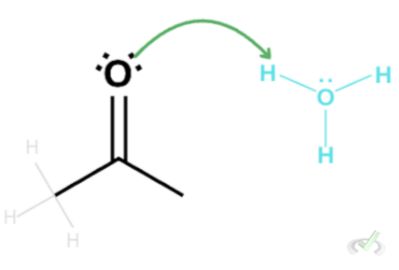
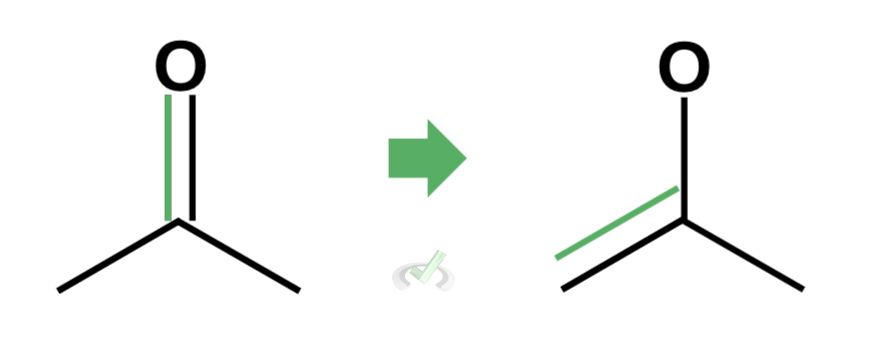
We do this because the end point of tautomerization is the formation of an alcohol with a double bond. By removing the double bond on the carbonyl atom, we are allowing another bond to form with oxygen.
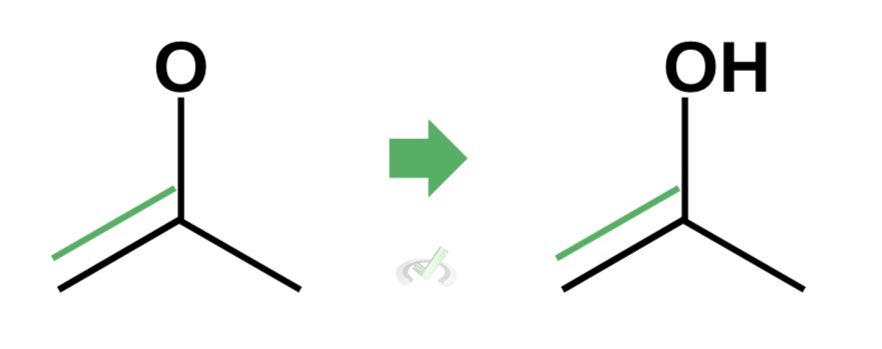
Finally, we can write the hydrogen atom next to the oxygen to complete the enol.
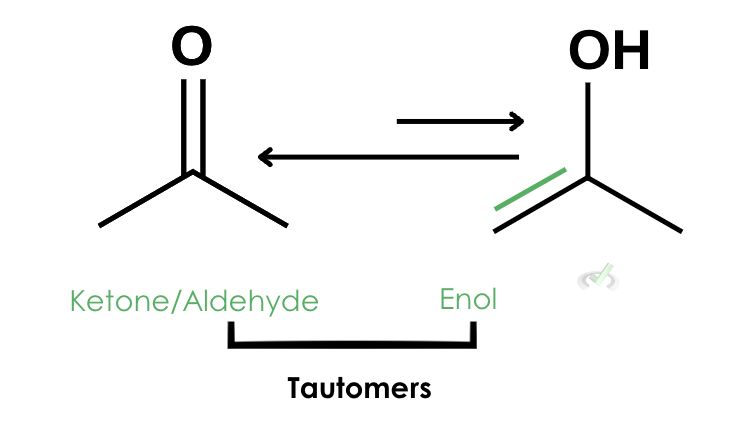
As you can see, the transformation of ketone into an enol is represented by two arrows with the arrow pointing towards the keto form being slightly longer. Although these reactions are in equilibrium, the keto form is favored because its structure is much more stable than the enol form.
The carbonyl (C=O) bond is much stronger than a carbon-carbon (C=C) bond. Since oxygen is more electronegative, the distribution of electrons around the oxygen atom offers more stability. Another factor is the intramolecular hydrogen bond that forms between the oxygen and the hydrogen from the surrounding solution.
We call the keto and enol forms tautomers. Tautomers are compounds that only differ in the arrangement of atoms. They are in equilibrium which means they can transform constantly in the presence of a catalyst.Most tautomerization reactions favor the formation of its keto form. Think of it this way: if we have a jar of aldehydes or ketones and we put an acid or base as a catalyst, there will always be an enol present in this jar since they are in equilibrium. Since ketos have a much more stable form, there will only be a small amount of enols present.
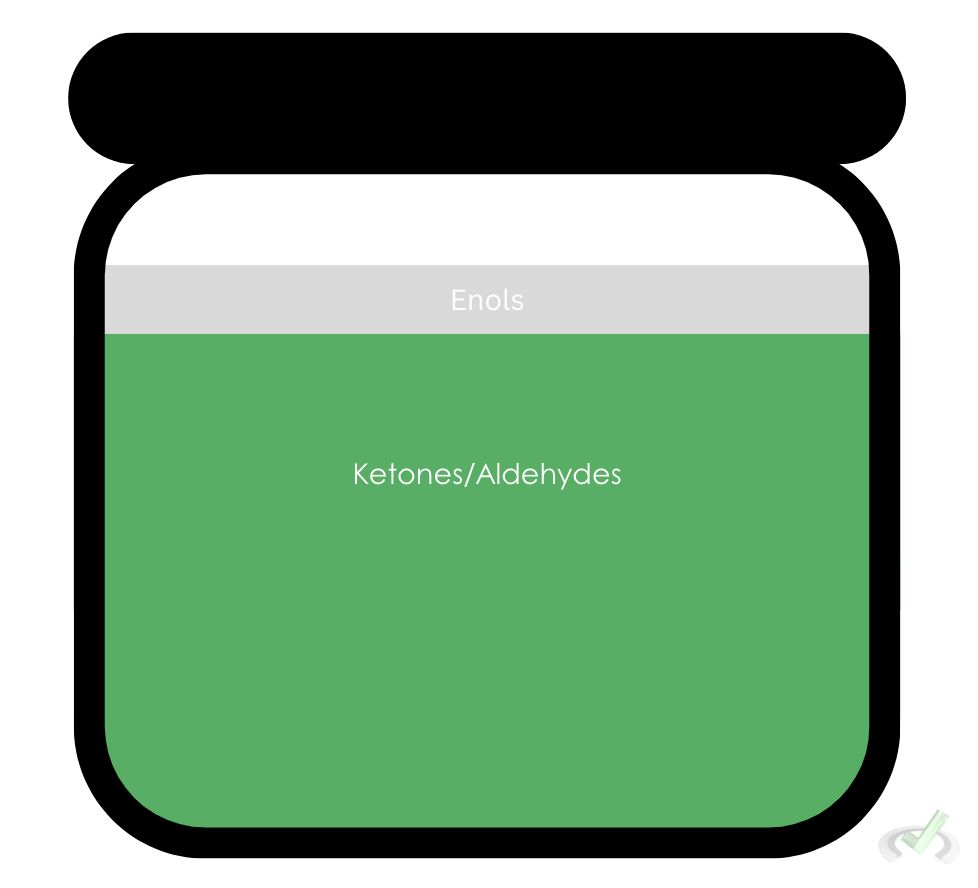
Now that we’ve established what tautomerization is, let’s move on to see how tautomerization happens in other environments
II. Keto-Enol Tautomerization in Acidic Condition
Acids and Bases have different definitions based on whatever reference we use. The Brønsted-Lowry definition of acids and bases recognizes a proton donor as an acid and a proton acceptor as a base. For tautomerization in an acidic solution, we use the hydronium ion (H3O+) as the acidic catalyst since it donates a proton.
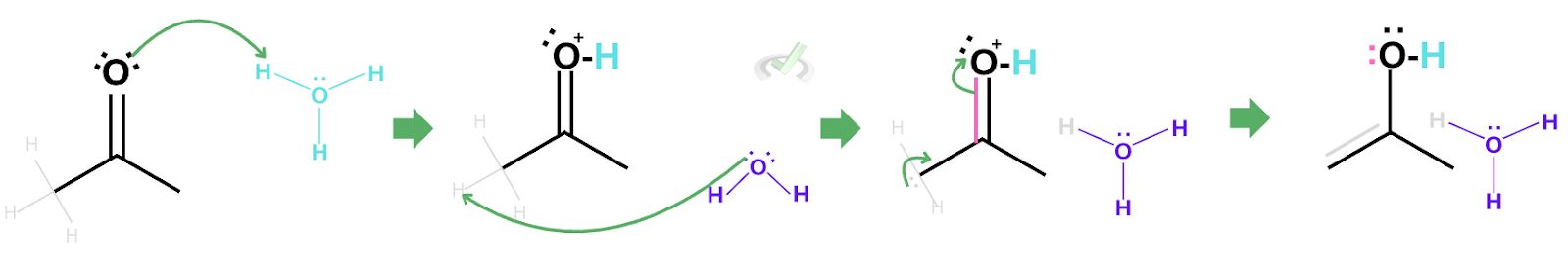
The first step of tautomerization is the protonation of the carbonyl oxygen. In this process, the partially negative electrons from the oxygen take a partially positive hydrogen atom found in the hydronium ion. As this happens, hydrogen breaks its bond with oxygen since hydrogen can only form one bond.

Oxygen now bears a partially positive charge. This makes it quite unstable since oxygen is electronegative.
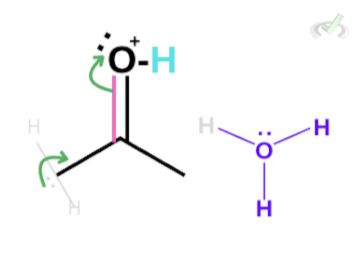
Since carbon is partially positive and we have hydrogen bonded to this carbon, the electrons in hydrogen are now heavily attracted to the partially positive carbon. Lucky for us, since we have a water molecule in the surroundings, the lone pair of electrons on the oxygen can bond with hydrogen which will cause the electrons to push towards carbon. This also makes the electrons on the double bond collapse into the oxygen atom. This is a chain reaction that results in the formation of an enol and a hydronium ion.
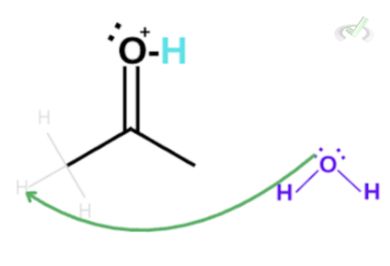
Notice how the hydronium ion at the beginning of the reaction is still present? Although this hydronium ion is not the same as the one at the start of the reaction, we see that the number of atoms involved is equal. Since the hydronium ion helped the reaction to take place without getting used up, the hydronium ion acted as a catalyst.
III. Keto-Enol Tautomerization in Basic Condition
In a base-catalyzed tautomerization reaction, we use the hydroxide ion (OH-) to catalyze the reaction.
The hydroxide ion has a negative charge and three lone pairs. These lone pairs can take the hydrogen, causing the electrons to collapse into the carbon-carbon (C-C) bond. Since carbon can only hold four bonds, a bond in the carbonyl atom will collapse into the oxygen atom.
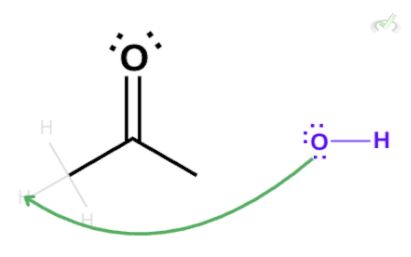
This results in an intermediate form where oxygen is bonded to carbon in a single bond with a negative charge and a carbon-carbon (C-C) double bond. The oxygen is still unstable in this state since it bears a negative charge. Again, it will take another hydrogen from a surrounding water molecule and form a bond with it, creating a hydroxyl group.
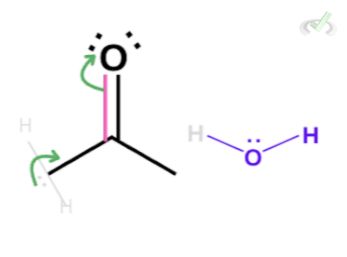
What we end up with is the enol form of our aldehyde or ketone. Similar to the reaction in an acidic medium, we still had the same number of hydroxide ions that we started with. Since it helped the reaction take place without being used up, the hydroxide ion acted as a catalyst for the reaction.
IV. The Reverse of Keto-Enol Tautomerization
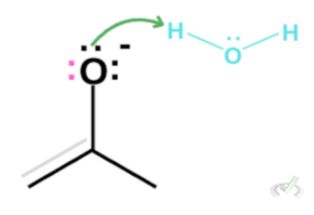
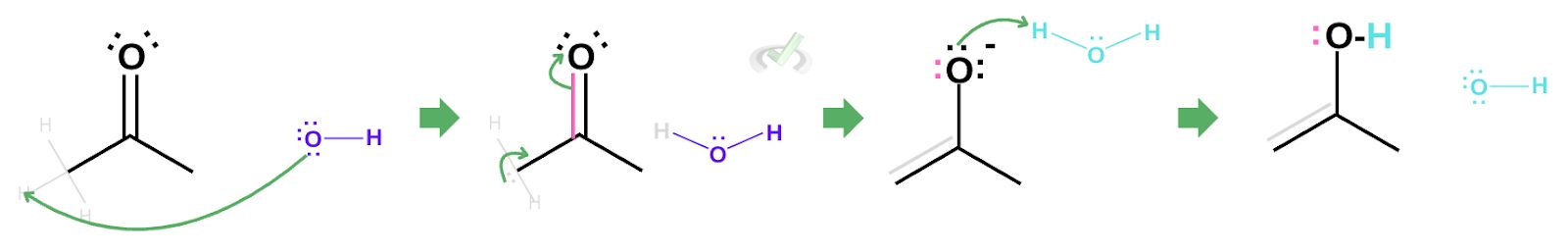
Since our reaction is represented by two arrows pointing at either side, this means an enol can revert back into its keto form. Consider an enol in an acidic environment.
The oxygen, in this case, still has two lone pairs. This lone pair can create another bond between oxygen and carbon. The electrons in the (C-C) double bond will be kicked out to satisfy the octet rule, taking hydrogen from a surrounding hydronium.
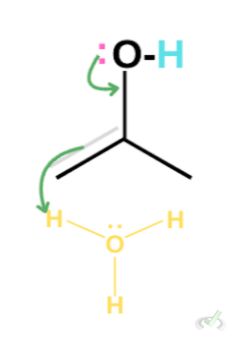
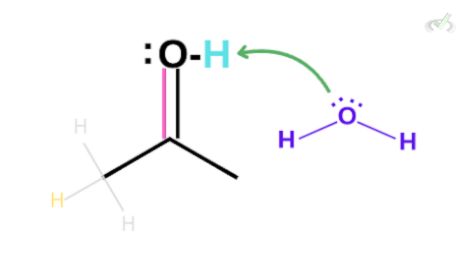
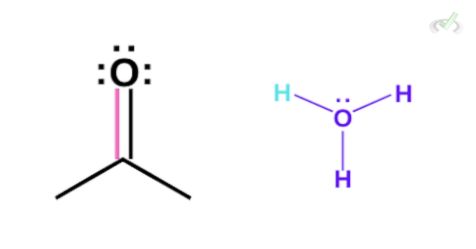
Again, this leaves the oxygen to be partially positive. Since this is still not neutral, the oxygen from a surrounding water molecule can grab the hydrogen which leaves our final keto product.
Note that since we have two arrows with different lengths showing reversibility, the direction pointed at by the longer arrow is more favored.
This is because sometimes, the keto form is more stable than its enol form. However, this does not mean we will not have any enol present. This only means that most of the reaction will always lead to the formation of keto forms.
V. Other examples
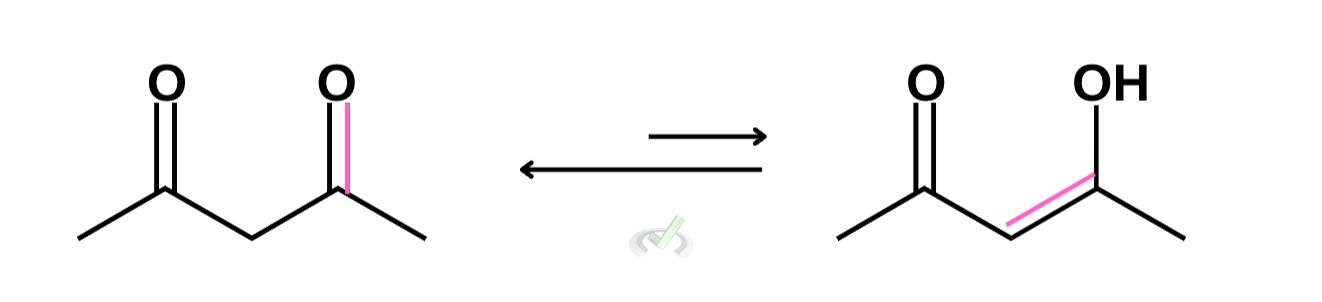
A. Tautomerization of acetylacetone
In this example, we can move any of the two double bonds in either direction. However, the structure will be more stable when double bonds are conjugated. The final step of showing tautomerization is by adding hydrogen to the carbonyl oxygen. The keto form is still much more stable than its tautomer so we denote this by making a longer arrow pointing to the left.
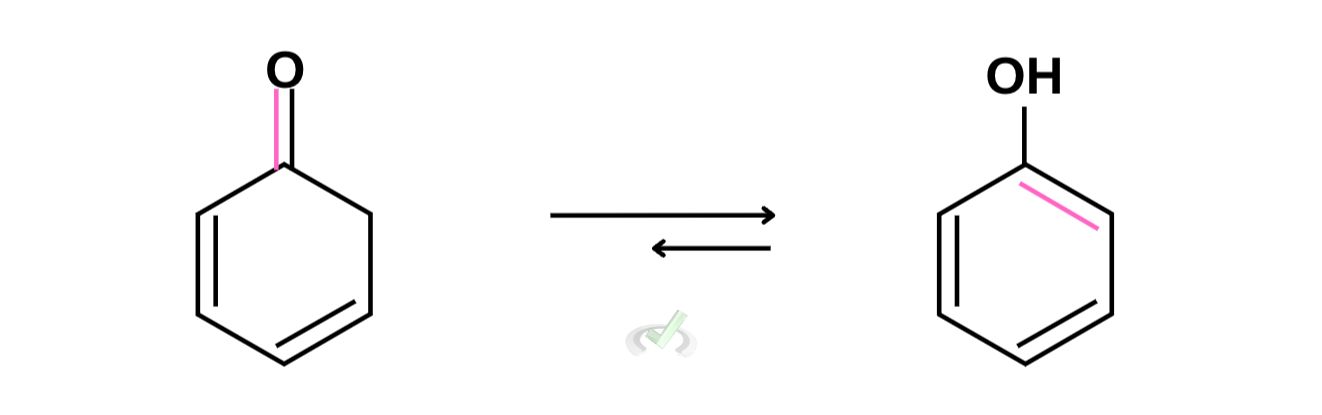
B. Tautomerization of cyclohexadienone
For this compound, we can remove the double bond from the carbonyl and move it to the remaining space available for bonding. We also have to add hydrogen to the carbonyl oxygen. Notice how this looks similar to an aromatic compound? That’s because it is! Aromatics are a lot more stable so for this reaction our arrow should be longer on the enol side to show that the equilibrium shifts more to the right.
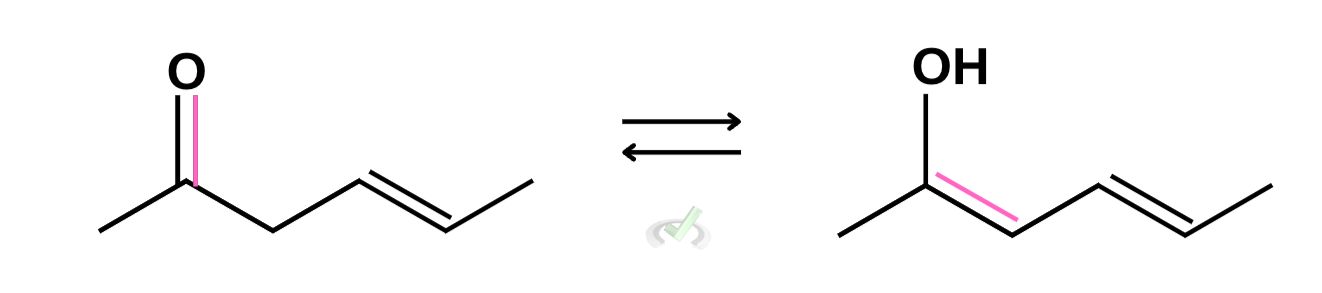
C. Tautomerization of 4-hexen-2-one
Similar to the previous examples, we can directly remove one bond from the carbonyl and place it conjugate to the other double bond. We also added hydrogen to signify the protonation of oxygen. In cases where we’re not certain which structure is favored or more stable, we can write arrows with the same length!
VI. Conclusion
Keto-enol tautomerization is a type of reaction for ketones and aldehydes that occurs in the presence of an acidic or basic catalyst. An enol tautomer will always have a double bond and an (-OH) group. They are isomers of ketones that are triggered by the presence of a proton donor or acceptor. First, the surrounding acid or base will take an alpha hydrogen and as this happens, the electrons left behind form a double bond between the two carbons. Since carbon can only hold four bonds, the electrons forming the double bond with the carbonyl atom will also collapse into the oxygen atom which will make the oxygen bear a negative charge. Lastly, due to this instability, the surrounding acid or base will donate hydrogen to the oxygen atom. Tautomerization reactions are reversible, however, some structures are more stable than others so the more stable structure is more favored. This means that the less-stable form will only be present in small amounts.
VII. Key Terms
- Aromatic Compounds - Organic compounds that have a cyclic conjugated structure. These compounds are stable due to their structure.
- Catalyst - A catalyst is any species that increases the rate of reaction without being consumed.
- Enol - A compound with a double bond and a hydroxyl group. These are isomers of aldehydes and ketones that undergo a special type of reaction.
- Hydronium ion - A cation that forms from the protonation of water and acts as Lewis acid.
- Hydroxide ion - An anion that carries a negative charge and acts as a Lewis base
- Octet Rule - Most atoms prefer to have eight electrons in their outer shell. One lone pair is equivalent to two electrons that make one bond.
- Tautomerization - The protonation at one molecular side and deprotonation at another.
VIII. Practice Questions
Sample Practice Question 1
Which of the following is the best enol form of this compound?
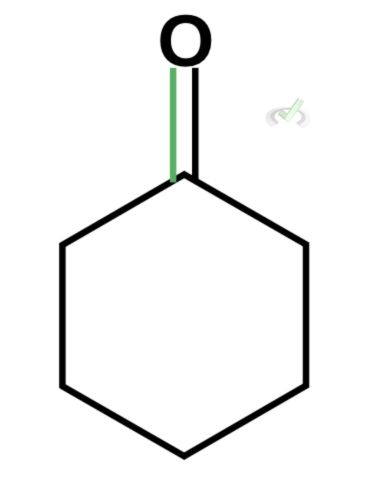
A.
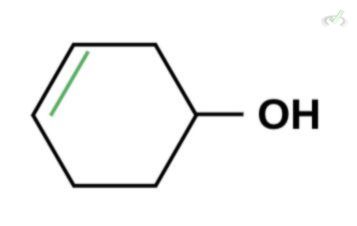
B.
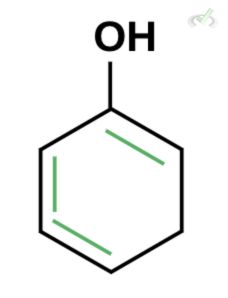
C.
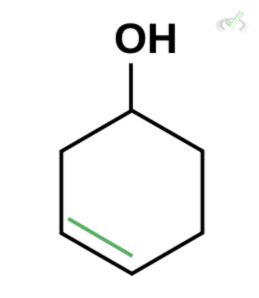
D.
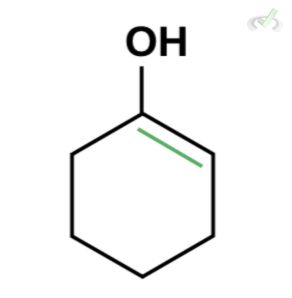
Ans. D
Sample Practice Question 2
Which of the following does not accurately describe the mechanism of keto-enol tautomerization?
A. A surrounding proton acceptor will take an alpha hydrogen from the alpha carbon.
B. The electrons will form a C=C double bond as a result of the deprotonation.
C. The carbonyl carbon has a double bond with oxygen and another double bond with the deprotonated carbon.
D. A proton donor will donate hydrogen to the negatively charged oxygen.
Ans. C







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
