Carboxylic acids are organic compounds with a functional group that can act as a leaving group. Compared to aldehydes and ketones that have hydrogen or another functional group at the carbonyl carbon, carboxylic acids have good leaving groups that allow them to undergo a special mechanism that allows the formation of new compounds known as derivatives.
In this lesson, we’ll talk about this mechanism known as nucleophilic substitution reactions that allow the formation of derivatives. These derivatives are known as acid halides, amides, acid anhydrides, esters, and nitriles. We’ll take a look at how one mechanism differs from the other and how its synthesis works.I. Nucleophilic Substitution: Addition–elimination process
Nucleophilic substitution reactions may sound familiar from nucleophilic addition reactions in aldehydes and ketones. Both reactions begin with a nucleophile attacking an electrophile. The difference is in the process following nucleophile attack.
- Nucleophilic addition reactions only rearrange the atoms in a compound while adding a nucleophile, without removing any original atoms.
- Nucleophilic substitution reactions, however, involve the displacement of a leaving group when the nucleophile attacks an electrophilic carbon.
General Mechanism:
- Nucleophilic Addition: The nucleophile attacks the carbonyl carbon, causing the electrons from the double bond to move back to the oxygen, creating a tetrahedral intermediate.
- Elimination: The oxygen reforms the carbonyl bond by pushing electrons back to the carbon, displacing the leaving group and forming a new acyl compound.
II. Reactivity of Carboxylic Acid Derivatives
The reactivity of carboxylic acid derivatives follows this order:
Acid halide > Acid anhydride > Ester > Amide
Less reactive derivatives can be synthesized from more reactive ones through nucleophilic substitution reactions.
III. Synthesis of Carboxylic Acid Derivatives
1. Acid Halides
Acid halides can be prepared from carboxylic acids using thionyl chloride (SOCl𝟯), phosphorus trichloride (PCl𝟯), or phosphorus tribromide (PBr𝟯).
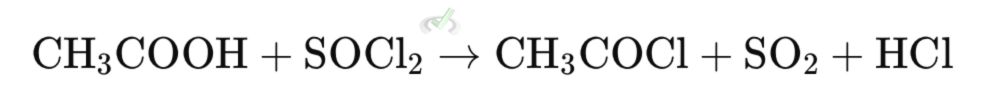
Example: Preparation of Acetyl Chloride from Acetic Acid
- Nucleophilic Addition: Oxygen from the -OH group attacks the partially positive sulfur atom in SOCl𝟯, ejecting chloride ion.
- Elimination: The chloride ion attacks the carbonyl carbon, displacing the leaving group.
2. Acid Anhydrides
Acid anhydrides can be prepared by the condensation of two carboxylic acids with water loss:
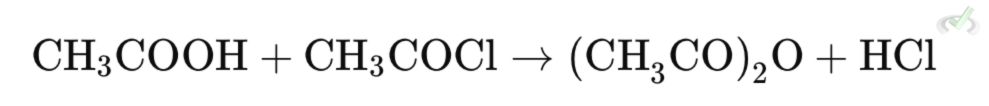
Or by the reaction of carboxylic acid with an acid halide:
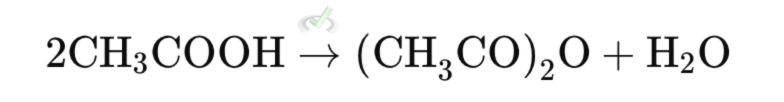
- Nucleophilic Addition: The carboxyl oxygen attacks the carbonyl carbon of the acid chloride.
- Elimination: The chloride ion is displaced as the leaving group.
3. Esters
Esters are synthesized through esterification, where a carboxylic acid reacts with alcohol in the presence of acid catalyst.
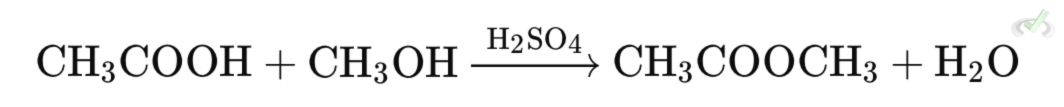
- Nucleophilic Addition - The alcohol attacks the protonated carbonyl carbon, forming a tetrahedral intermediate.
- Elimination - The oxygen reforms the carbonyl bond, ejecting water as the leaving group.
4. Amides
Amides are synthesized from acid derivatives using nucleophilic substitution reactions with ammonia or amines.
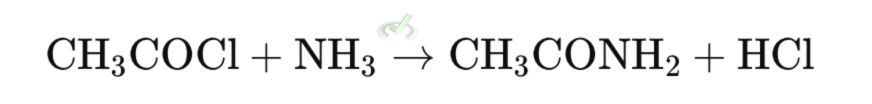
- Nucleophilic Addition - The nitrogen from methylamine attacks the carbonyl carbon.
- Elimination - Oxygen reforms the carbonyl, ejecting water as a leaving group.
IV. Conclusion
Carboxylic acids are highly reactive due to their functional group, allowing nucleophilic substitution reactions to form derivatives. These reactions follow an addition-elimination mechanism, leading to the formation of acid halides, acid anhydrides, esters, and amides.
VII. Key Terms
- Carboxylic Acid Derivatives - Functional groups that arise from carboxylic acids through nucleophilic substitution reactions.
- Nucleophilic Substitution - A reaction that involves a nucleophilic attack on an electrophile that triggers the release of a leaving group.
- Leaving group - An atom or group of atoms that leaves a parent molecule in a chemical reaction.
- Good leaving groups: stable and neutral after dissociation.
- Poor leaving groups: do not readily dissociate.
VIII. Practice Questions
Sample Practice Question 1
The following statements accurately describe reactions of carboxylic acids or their derivatives EXCEPT:
A. Elimination follows after nucleophilic addition.
B. The reactivity of carboxylic acid derivatives can be described as Acid halide > Acid anhydride > ester > amide.
C. During nucleophilic substitution, the nucleophile ejects itself as a leaving group.
D. Acid anhydrides can be prepared by the condensation of two carboxylic acids.
Ans. C
In nucleophilic substitution, the nucleophile replaces a leaving group, not ejects itself. The leaving group is a different part of the molecule that detaches after the nucleophilic attack.
Sample Practice Question 2
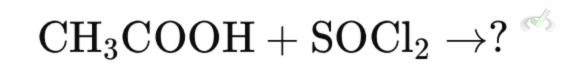
What is the major product of this reaction?
A. Acid halide
B. Amide
C. Ester
D. Acid anhydride
Ans. A
The reaction of carboxylic acids with thionyl chloride (SOCl₂) produces an acid chloride (acyl chloride). In this case, acetic acid (CH₃COOH) reacts with SOCl₂ to form acetyl chloride (CH₃COCl), sulfur dioxide (SO₂), and hydrogen chloride (HCl).







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
