In our article on nucleophilic substitution reactions of carboxylic acids, we discussed how carboxylic acids undergo substitution reactions to form carboxylic acid derivatives. This reaction involves two key steps: addition and elimination.
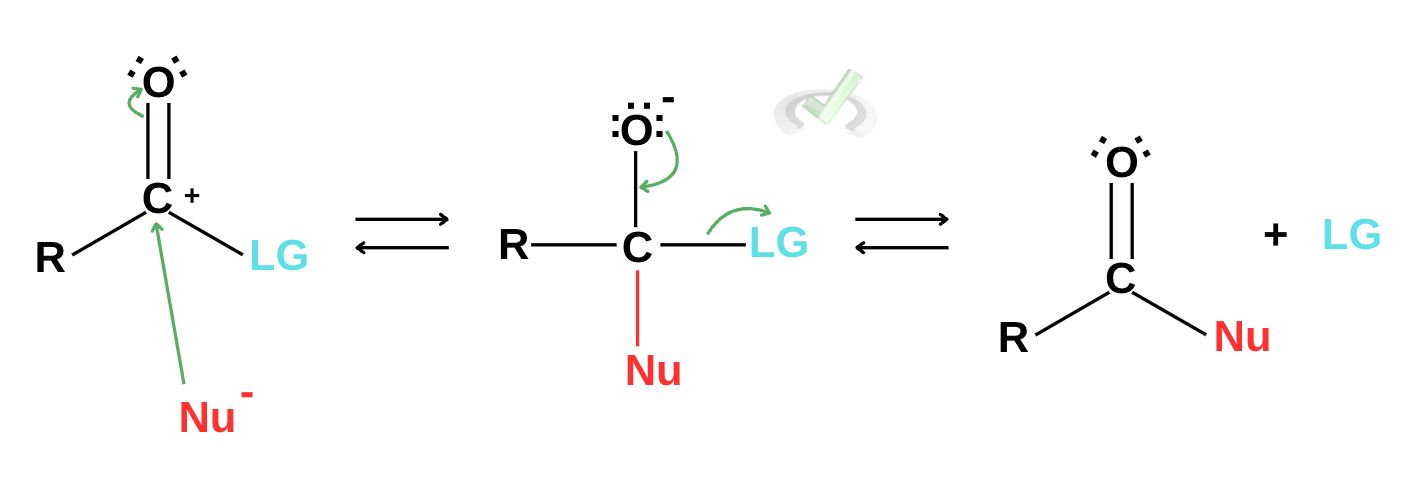
In nucleophilic addition, a nucleophile attacks the partially positive carbonyl carbon. This will rearrange the atoms and effectively remove a good leaving group as part of the elimination process. The nucleophile essentially substitutes the leaving group, forming a new compound.
We also talked about reactivities and learned about the relative reactivity of these derivatives. Here’s a summary of carboxylic acids and their order of reactivity from most to least reactive.
Carboxylic acids and their derivatives are mostly known for these substitution reactions at the acyl (carbonyl) atom. In this article, we’ll learn about these substitution reactions that occur in carboxylic acid derivatives.
I. Hydrolysis
When we say that something undergoes hydrolysis, we refer to adding water to something. Hydrolysis is a chemical reaction wherein a compound reacts with water. When carboxylic acid derivatives undergo hydrolysis, they react with water to form carboxylic acids.
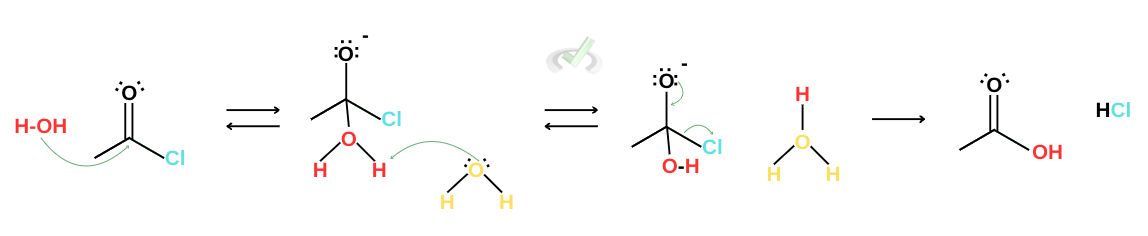
During hydrolysis, nucleophilic addition begins when a water molecule acts as a nucleophile and attacks the carbonyl carbon, forming a tetrahedral intermediate. A surrounding water molecule will act as a base and protonate using the water molecule attached to the carbon. This is followed by elimination, where the electrons rearrange to eject the good leaving group, the chloride ion. Chloride ions can protonate from surrounding water molecules, forming hydrochloric acid and the product carboxylic acid.
A. Acyl Halide
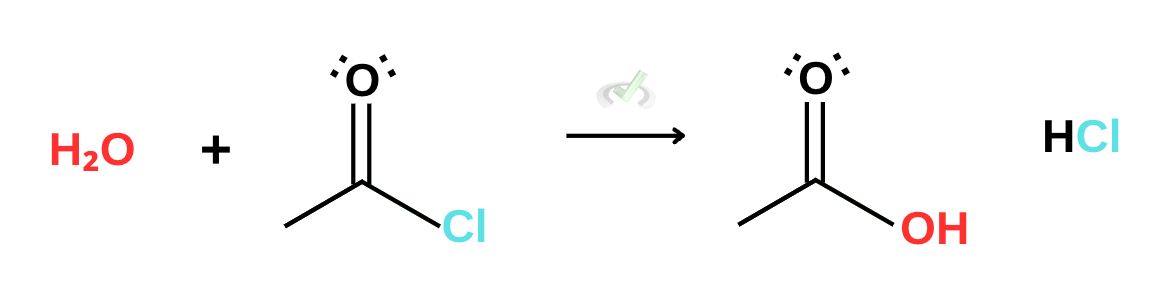
The same nucleophilic addition-elimination happens for all carboxylic derivatives. It’s important to note, however, that the reactivities of components matter here. We previously said that acyl halides are the most reactive derivative; when something is more reactive than the other, the reaction moves towards the less reactive component. This is still a part of Le Chatelier’s principle. Since acyl halides are more reactive than carboxylic acids, the reaction moves towards producing carboxylic acids. This reaction is product-favored.
B. Acid Anhydride
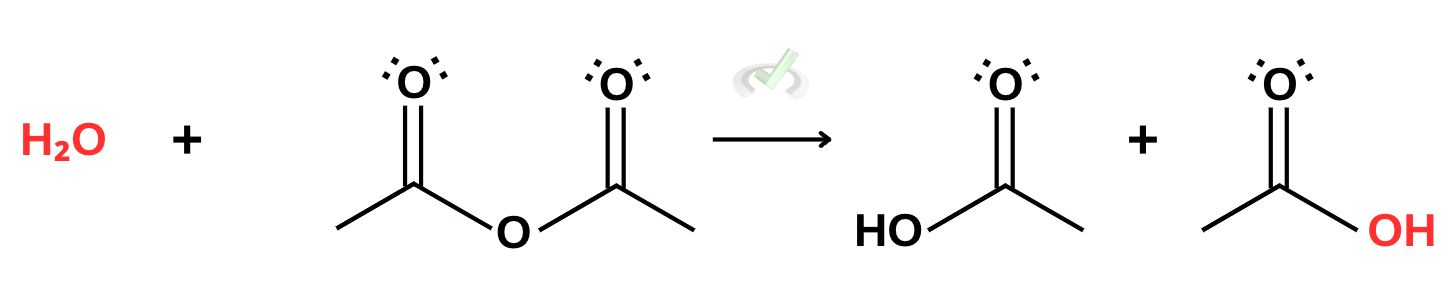
Since synthesizing anhydrides involves removing water, adding water to anhydrides will break the anhydride into its original components. This reaction is another product favorite reaction since anhydrides have greater reactivity than carboxylic acids.
C. Ester
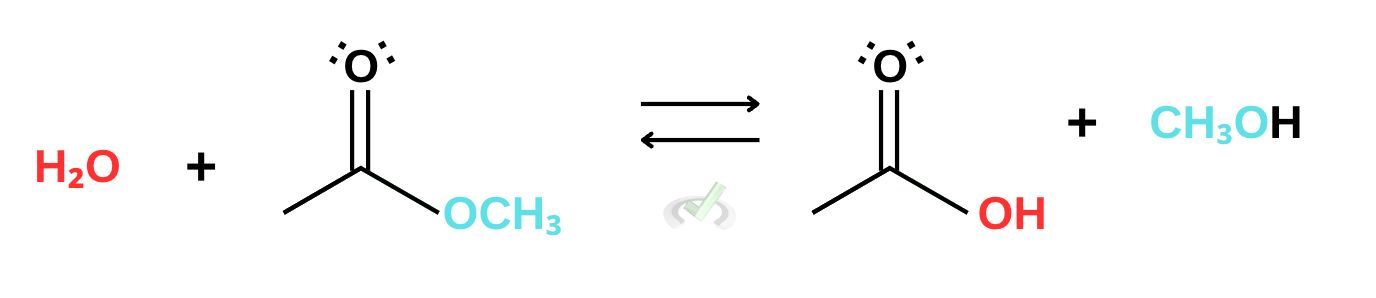
Similar to anhydrides, since ester synthesis involves the reaction between alcohol and carboxylic acids, hydrolysis breaks esters down into alcohol and carboxylic acid. Notice that the reaction is in equilibrium. This reversibility is due to the similar reactivities of carboxylic acid and esters. Because they have similar reactivities, this reaction can happen simultaneously.
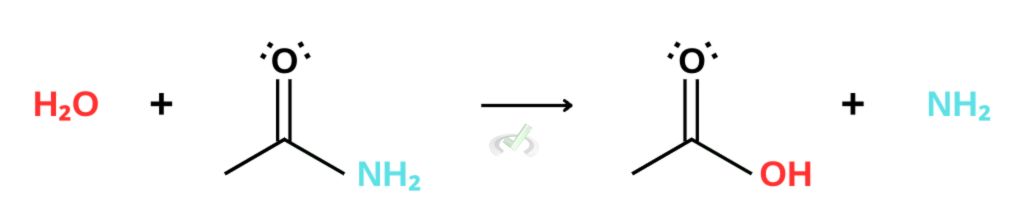
D. Amides and Nitriles
Amides
Nitriles
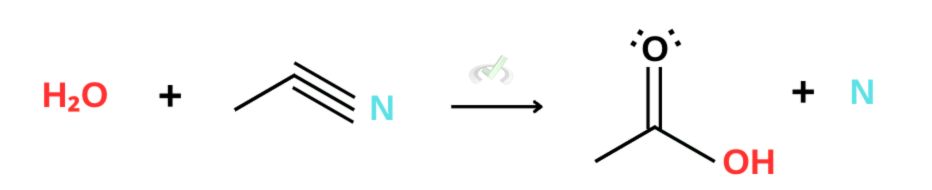
Referring to our reactivity list, we see that amides and nitriles are less reactive than carboxylic acid. This makes hydrolysis difficult for nitriles and amides under neutral conditions. Hydrolysis for these derivatives requires using a strong acid and a strong base as catalysts for the reaction.
II. Transesterification
Transesterification is a chemical reaction where an ester replaces another ester in the presence of alcohol under acidic or basic conditions. In this reaction, an ester replaces another.
Basic Conditions
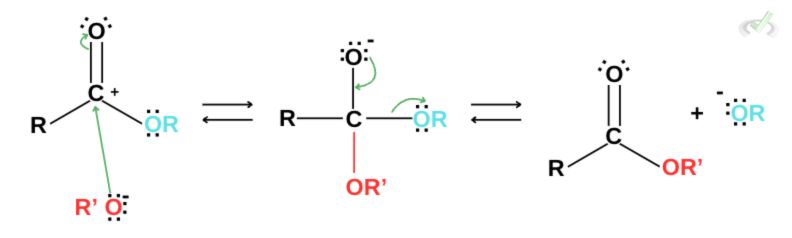
Under basic conditions, the reaction also follows the same addition-elimination reaction. An alkoxide initially acts as a nucleophile and attacks the partially positive carbon. This displaces the electron arrangement and triggers the removal of a good leaving group. For transesterification, the leaving group is another ester. The reaction completes once the nucleophile attaches and an ester leaves as a good leaving group.
Acidic Conditions
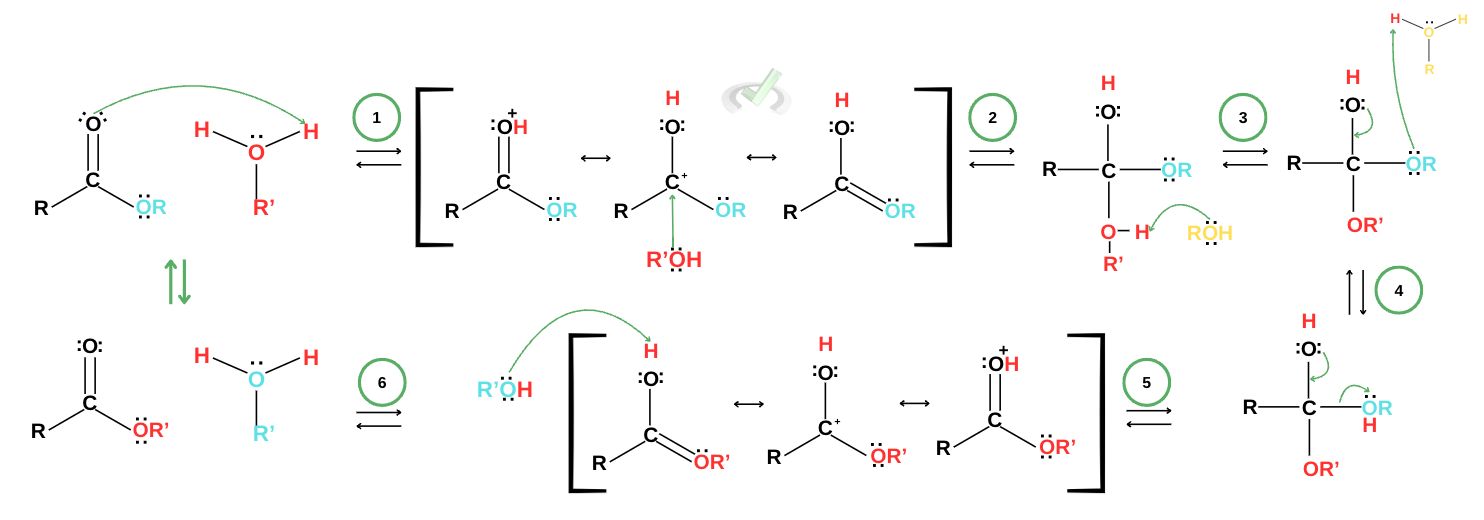
For transesterification in acidic conditions, the reaction takes a six-step process.
A protonated alcohol group protonates the oxygen to make a resonance-stabilized ion.
The alcohol acts as a nucleophile and attacks the ion forming a tetrahedral intermediate.
Ester group protonates.
Electrons rearrange to eject the ester as a leaving group.
The leaving group protonates from the resonance-stabilized ion.
Alcohol removed acts as a base, completing the reaction.
III. Conclusion
Transesterification and hydrolysis are reactions that carboxylic acid derivatives go through to get specific products. Both reactions involve nucleophilic substitution reactions that involve adding a nucleophile and eliminating a good leaving group. Transesterification refers to the chemical reaction that occur when an ester replaces another ester group in the presence of alcohol under basic or acidic conditions. Hydrolysis, on the other hand, is a reaction that involves reacting a derivative with water to form carboxylic acids.
IV. Key Terms
- Alkoxide - the conjugate base of an alcohol composed of a negatively charged oxygen bonded to an organic group.
- Hydrolysis - a chemical reaction that involves water as a reactant.
- Nucleophilic substitution - a type of reaction wherein a nucleophile attacks an electrophile, eliminating another component.
- Reactivity - how easily something undergoes a chemical reaction.
- Transesterification - a chemical reaction that involves an ester replacing another ester in a compound.
V. Practice Questions
Sample Practice Question 1
Which of the following reactions is a reactant-favored reaction?
A. Acid chloride + water
B. Acid anhydride + ester
C. Acid chloride + ester
D. Carboxylic acid + carboxylic acid
Ans. D
Sample Practice Question 2
In the figure below, sodium hydride acts as the
A. Catalyst
B. Electrophile
C. Nucleophile
D. Base
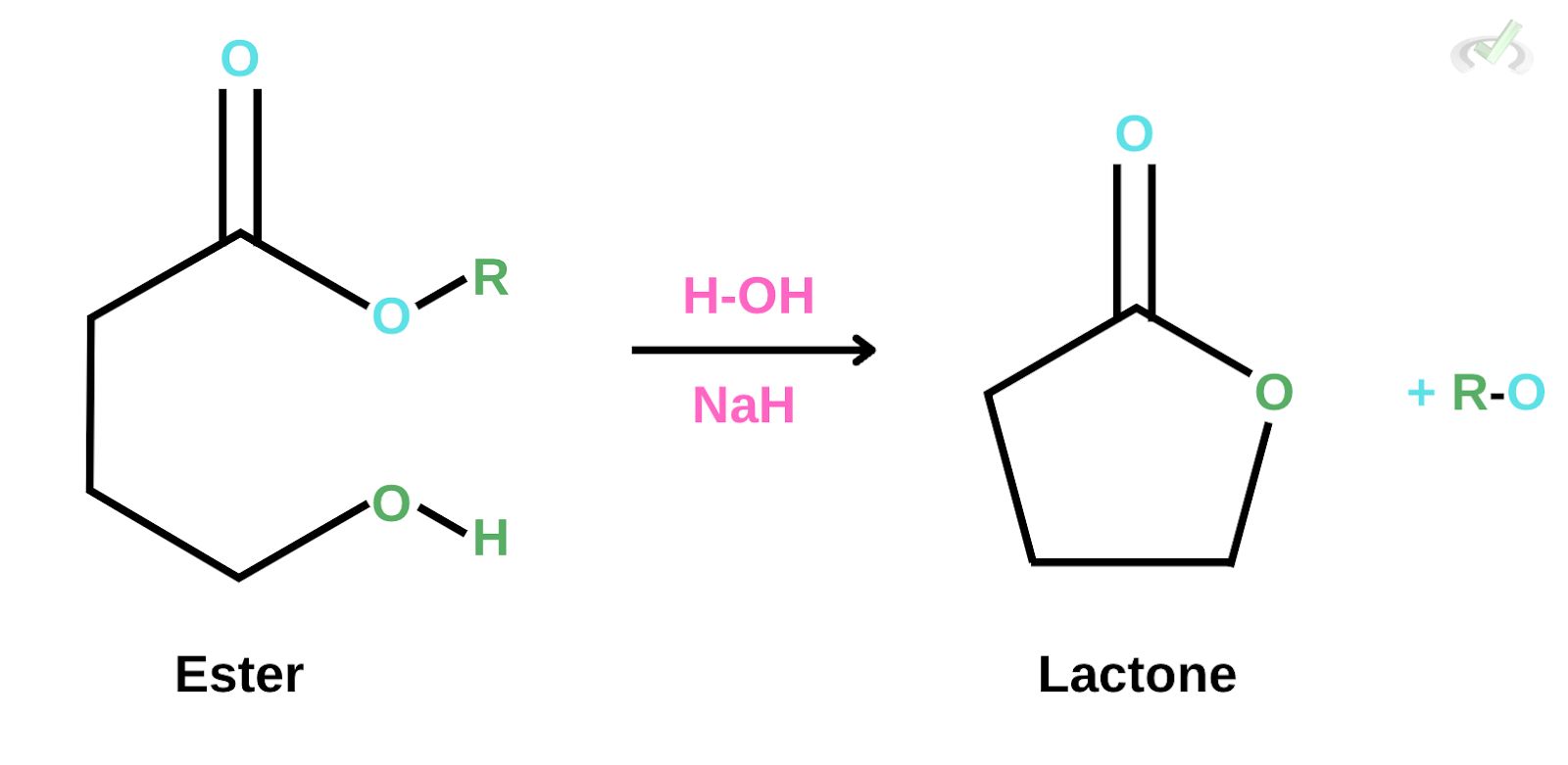
Ans. C







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
