Nucleophilic substitution reactions are an important topic in organic chemistry. They involve replacing a leaving group (usually a halogen) with a nucleophile (a molecule or ion with a lone pair of electrons). Understanding these reactions is key to mastering many concepts in organic chemistry.
I. Basics of Nucleophilic Substitution
A. What is a Nucleophile?
A nucleophile is a molecule or substance that donates an electron pair in order to form a chemical bond. Nucleophiles can be negatively charged ions like hydroxide ions (OH-) and cyanide ions (CN-).
They can also be neutral molecules like ammonia (NH3). Strong nucleophiles react quickly and easily, while weak nucleophiles react more slowly.
B. What is a Leaving Group?
A leaving group is an atom or group that can depart with an electron pair. This forms a stable ion or molecule.
Common leaving groups are halides like chloride (Cl-), bromide (Br-), and iodide (I-). Good leaving groups are stable independently, making it easier for the nucleophile to attack.
II. Types of Nucleophilic Substitution
Nucleophilic substitution reactions have two main types, namely SN1 and SN2 reactions. Let’s explore them in details.
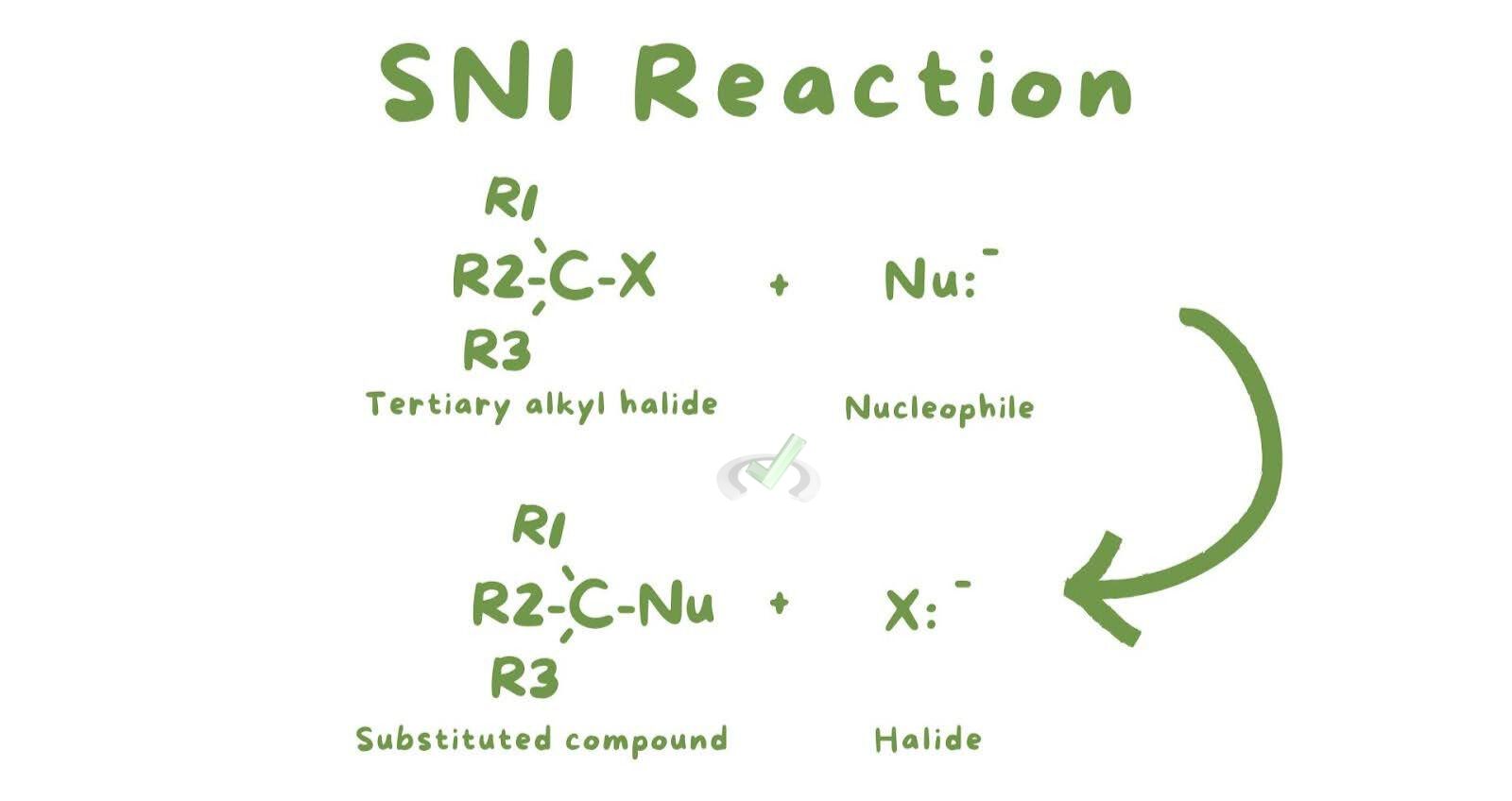
A. SN1 Reactions
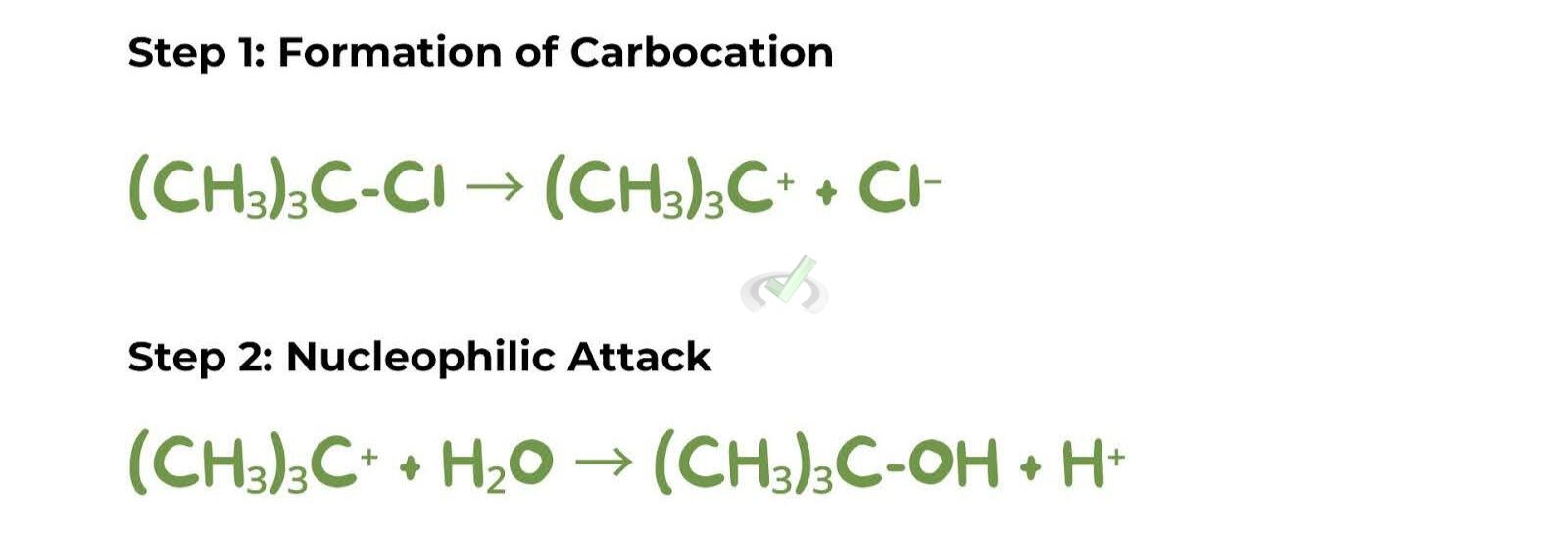
Mechanism
The SN1 mechanism occurs in two steps. First is carbocation formation: the leaving group departs, forming a positively charged carbocation. This step is the rate-determining step because it is the slowest. Second is the nucleophilic attack: the nucleophile attacks the carbocation, forming the product.
B. SN2 Reactions
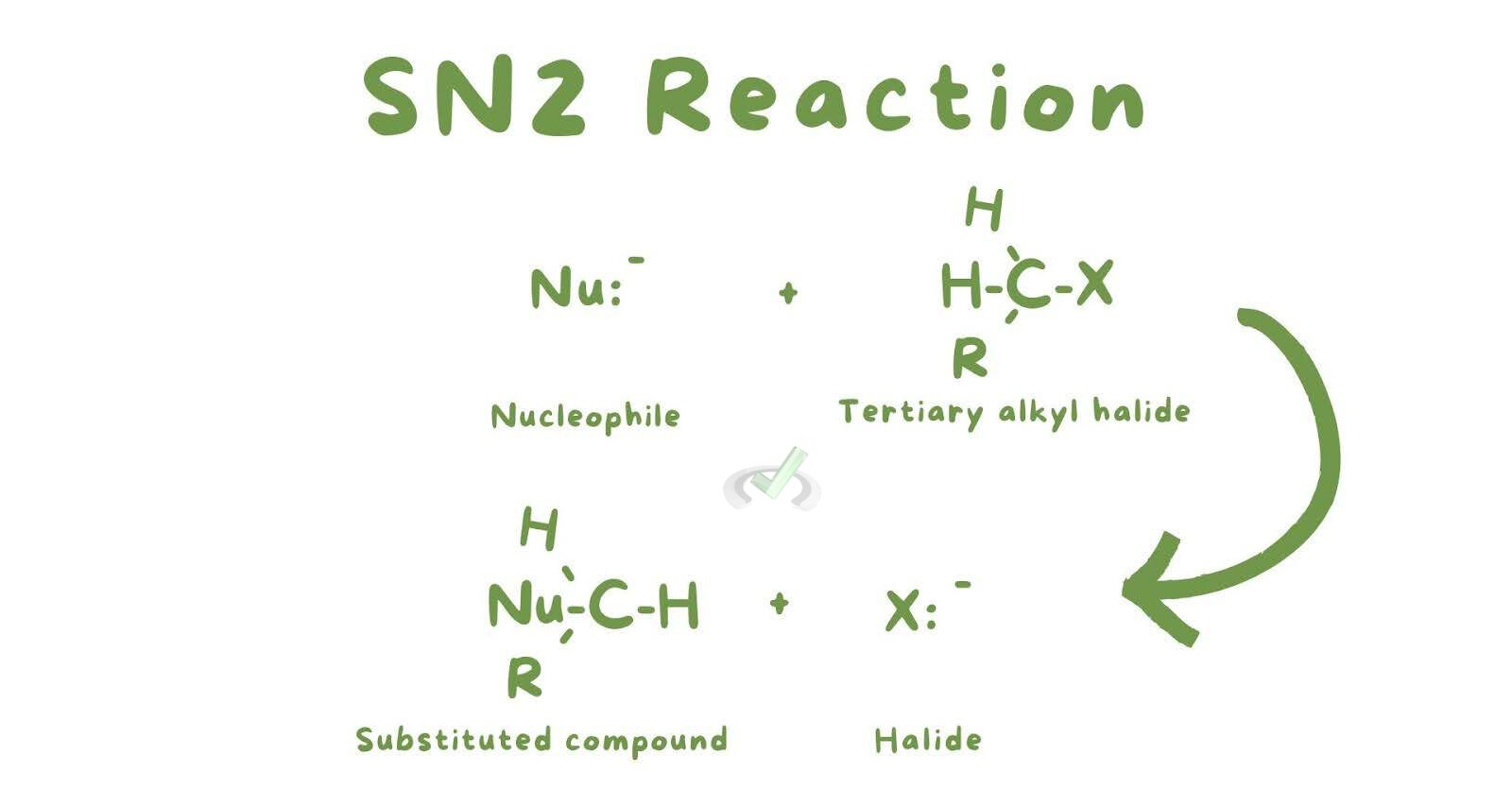
Mechanism
The SN2 mechanism occurs in a single, concerted step. The nucleophile attacks the substrate from the opposite side of the leaving group. This pushes the leaving group out.
Characteristics
The rate law for SN1 reactions depends only on the concentration of the substrate: Rate = k[substrate]. Tertiary carbocations are more stable and thus more likely to undergo SN1.
Stability is due to electron-donating groups around the carbocation. Polar protic solvents (like water and alcohol) stabilize the carbocation, favoring SN1.
The planar structure of the carbocation allows the nucleophile to attack from either side. This often leads to a racemic mixture of products.
Example
An example is the conversion of tert-butyl chloride (C(CH3)3Cl) to tert-butyl alcohol (C(CH3)3OH) using water as the nucleophile.
III. Factors Affecting Nucleophilic Substitution
A. Substrate Structure
In SN1 reactions, more stable carbocations favor the reaction.
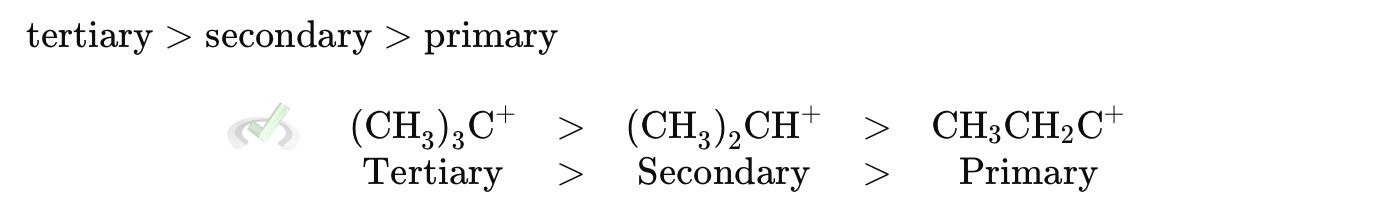
In SN2 reactions, less steric hindrance favors the reaction. Bulky groups around the carbon being attacked slow down SN2 reactions.
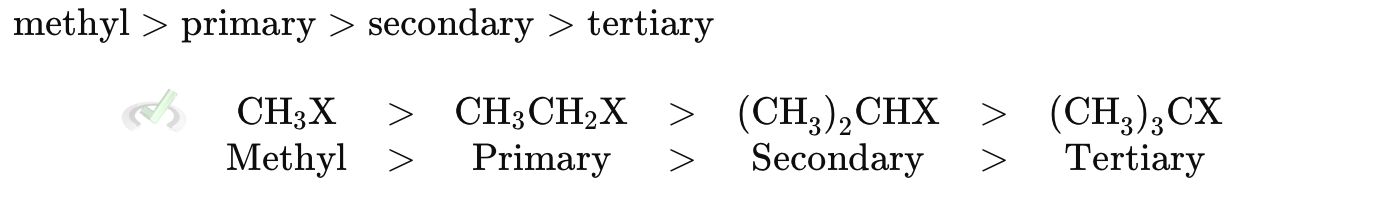
B. Nucleophile Strength
Strong nucleophiles (like OH-, CN-, and NH3) favor SN2. Weak nucleophiles (like water and alcohol) are more likely to participate in SN1.
C. Leaving Group Ability
Good leaving groups (like I-, Br-, Cl-) facilitate both SN1 and SN2 reactions. A good leaving group is stable once it has left the substrate.
D. Solvent Effects
Polar protic solvents stabilize the carbocation intermediate in SN1 reactions. Polar aprotic solvents do not solvate the nucleophile strongly in SN2 reactions, making it more reactive.
IV. Nucleophilic Substitution Applications and Examples
A. Synthesis of Alcohols
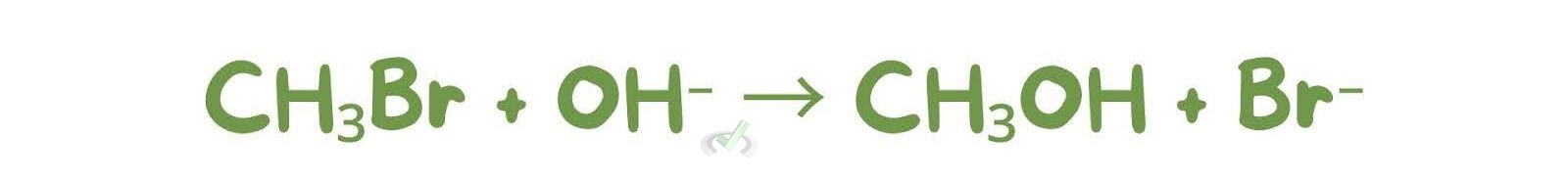
SN2 reactions can convert alkyl halides to alcohols using hydroxide ions. SN1 reactions can convert tertiary alkyl halides to alcohols using water or alcohol as the nucleophile.
B. Pharmaceutical Synthesis
Many drugs are synthesized using nucleophilic substitution reactions, such as the production of penicillin derivatives.
C. Biological Relevance
Nucleophilic substitution is crucial in biochemical processes. This includes DNA replication and enzyme activity.
V. Connecting to Broader Organic Chemistry Concepts
A. Enzyme Catalysis
Enzymes often use nucleophilic substitution in their catalytic cycles. Understanding this helps in studying enzyme mechanisms and drug design.
B. Drug Metabolism
Many drugs are metabolized in the liver through nucleophilic substitution reactions. This is important for understanding pharmacokinetics.
C. DNA and RNA Chemistry
Nucleophilic substitution plays a role in the replication and repair of DNA and RNA. This knowledge is crucial for genetics and molecular biology.
VI. Wrap-Up and Key Terms
Understanding nucleophilic substitution reactions is essential for learning many topics in organic chemistry. In these reactions, a leaving group is replaced by a nucleophile. There are two main types of mechanisms: SN1 and SN2.
The mechanism used depends on several factors, including the molecule's structure and the nucleophile's strength. The ability of the leaving group and the type of solvent also affect the mechanism.
Key Terms
- Nucleophile: A species that donates an electron pair.
- Leaving Group: An atom or group that departs with an electron pair.
- SN1 Reaction: A two-step nucleophilic substitution mechanism involving a carbocation intermediate.
- SN2 Reaction: A one-step nucleophilic substitution mechanism involving simultaneous bond formation and breaking.
- Steric Hindrance: When bulky groups around the reactive site slow the reaction.
- Racemic Mixture: A mixture of equal amounts of two enantiomers. These are molecules that are mirror images of each other.
VII. Practice Questions
Sample Practice Question 1
What is a nucleophile?
A) A species that accepts an electron pair.
B) A species that donates an electron pair.
C) A neutral molecule.
D) A positive ion.
Ans. B
A nucleophile is a species that donates an electron pair to form a chemical bond.
Sample Practice Question 2
Which solvent favors an SN2 reaction?
A) Water
B) Methanol
C) Acetone
D) Ethanol
Ans. C
Acetone is a polar aprotic solvent, which favors SN2 reactions.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
