In organic chemistry, redox reactions play a crucial role. "Redox" stands for reduction and oxidation. These reactions involve the transfer of electrons between molecules.
Hence, it changes the oxidation state of the molecules involved. Understanding redox reactions helps in studying many biological processes and industrial applications.
I. Basics of Redox Reactions
A. Oxidation
Oxidation is when a molecule loses electrons. When this happens, the oxidation state of the molecule increases.
For instance, when ethanol (C₂H₅OH) is converted to acetaldehyde (C₂H₄O), it loses hydrogen atoms. Losing hydrogen atoms means losing electrons, which is oxidation.
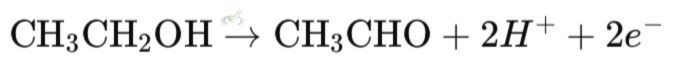
B. Reduction
Reduction is when a molecule gains electrons. When this happens, the oxidation state of the molecule decreases.
For example, when acetaldehyde (C₂H₄O) is converted to ethanol (C₂H₅OH), it gains hydrogen atoms. Gaining hydrogen atoms means gaining electrons, which is a reduction.
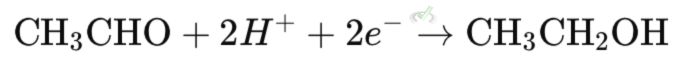
II. Common Redox Reactions in Organic Chemistry
A. Oxidation of Alcohol
Primary alcohols like ethanol can be oxidized to aldehydes like acetaldehyde. This happens when the alcohol loses hydrogen atoms.
Further oxidation of aldehydes leads to carboxylic acids like acetic acid. This happens when the aldehyde gains an oxygen atom.
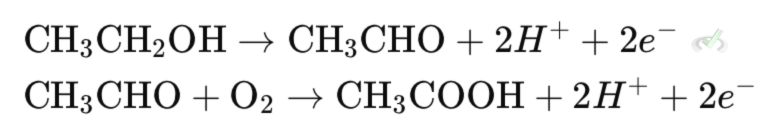
B. Reduction of Aldehydes and Ketones
Aldehydes like formaldehyde (CH₂O) can be reduced to primary alcohols like methanol (CH₃OH). This happens when the aldehyde gains hydrogen atoms.
On the other hand, ketones like acetone (C₃H₆O) can be reduced to secondary alcohols like isopropanol (C₃H₈O). This happens when the ketone gains hydrogen atoms.
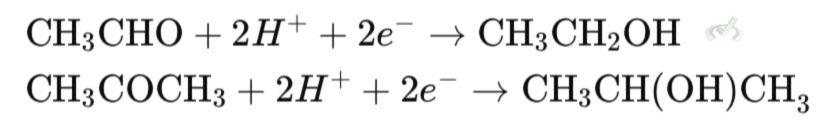
C. Oxidative Cleavage of Alkenes
Alkenes like ethylene (C₂H₄) can be broken down into smaller molecules using oxidizing agents. One example is ozonolysis, where ozone (O₃) breaks the double bond in ethylene. The process produces formaldehyde (CH₂O).
III. Agents Involved in Redox Reactions
A. Oxidizing Agents
Oxidizing agents gain electrons and get reduced in the process. They cause other molecules to lose electrons.
For example, potassium permanganate (KMnO₄) is a strong oxidizing agent. It oxidizes alkenes to diols (compounds with two -OH groups).
B. Reducing Agents
Reducing agents lose electrons and get oxidized in the process. They cause other molecules to gain electrons.
For example, lithium aluminum hydride (LiAlH₄) is a strong reducing agent. It is used to reduce aldehydes and ketones to alcohols.
IV. Applications of Redox Reactions
A. Biological Systems
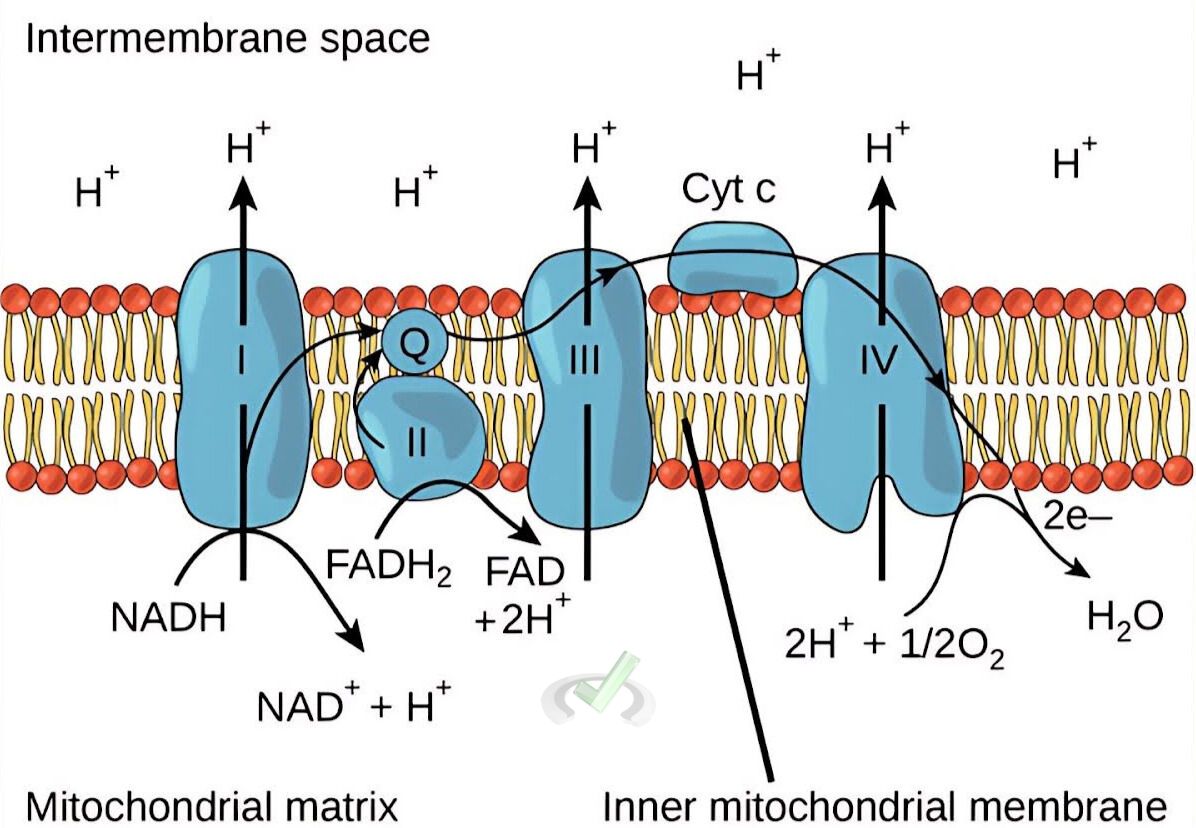
Redox reactions are vital in cellular respiration. In this process, glucose is oxidized to produce energy.
For example, electrons are transferred through a series of proteins in the electron transport chain. This transfer of electrons results in the production of ATP, the cell's energy currency.
B. Industrial Processes
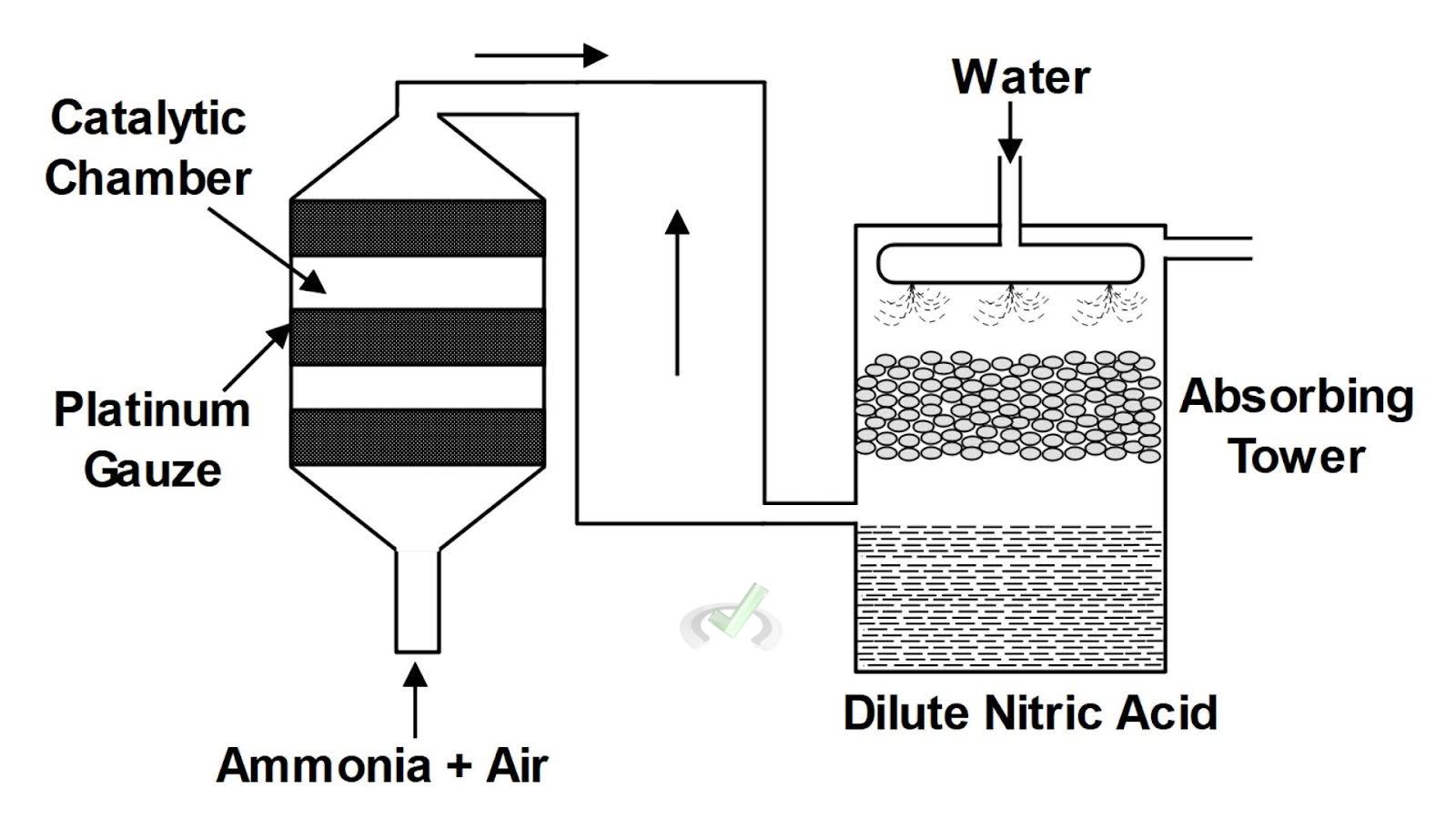
Redox reactions are used in the production of nitric acid. This process involves the oxidation of ammonia (NH₃).
For example, the Ostwald process uses a catalyst to oxidize ammonia to nitric oxide (NO). This nitric oxide is then further oxidized to nitrogen dioxide (NO₂). It reacts with water to form nitric acid (HNO₃).
V. Connecting Redox Reactions to Broader Organic Chemistry Concepts
A. Synthesis and Functional Group Transformations
Knowing redox reactions aids in designing synthetic pathways. For example, converting an alcohol to a carboxylic acid involves two oxidation steps.
First, the alcohol is oxidized to an aldehyde. And then, the aldehyde is further oxidized to a carboxylic acid.
B. Reaction Mechanisms
The behavior of molecules in redox reactions is influenced by their ability to transfer electrons. Hence, it's important for predicting reaction intermediates and transition states. For example, in the oxidation of ethanol to acetic acid, knowing the steps and intermediates helps understand the entire reaction mechanism.
C. Biochemical Relevance
Many biochemical pathways involve redox reactions. For example, the citric acid cycle includes multiple redox reactions that produce energy in the form of ATP.
Each step involves specific enzymes that facilitate the transfer of electrons. This is crucial for the cycle's function.
VI. Wrap-Up and Key Terms
Understanding redox reactions involves grasping several key concepts and principles. Let's review:
Key Terms:
- Oxidation: Loss of electrons, increase in oxidation state.
- Reduction: Gain of electrons, decrease in oxidation state.
- Oxidizing Agents: Substances that cause oxidation are reduced.
- Reducing Agents: Substances that cause a reduction, get oxidized.
VII. Practice Questions
Sample Practice Question 1
What happens to a molecule during oxidation?
A) It gains electrons.
B) It loses electrons.
C) Its oxidation state decreases.
D) It becomes reduced.
Ans. B
During oxidation, a molecule loses electrons, increasing its oxidation state.
Sample Practice Question 2
Which substance is a common oxidizing agent?
A) Lithium aluminum hydride (LiAlH₄)
B) Potassium permanganate (KMnO₄)
C) Methanol (CH₃OH)
D) Ethylene (C₂H₄)
Ans. B
Potassium permanganate is a strong oxidizing agent in various oxidation reactions.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
