Thermodynamics is the study of the relationship between energy and work. We study thermodynamics to gain a good understanding of energy, how it can be used to do work, and how it can be transformed from one form to another.
I. The Zeroth Law
It might sound odd to have a zeroth law; however, the order of these laws is crucial to understanding how they work in the bigger picture. This law is a fundamental law that was discovered after both the first and second laws were accepted. It serves as a law that formalizes the concepts of temperature and equilibrium.
This law states that:
“If two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.”
By definition, thermal equilibrium is the state where no movement of energy occurs between objects, when we say that two objects are in thermal equilibrium, no energy in any form moves or flows between them.
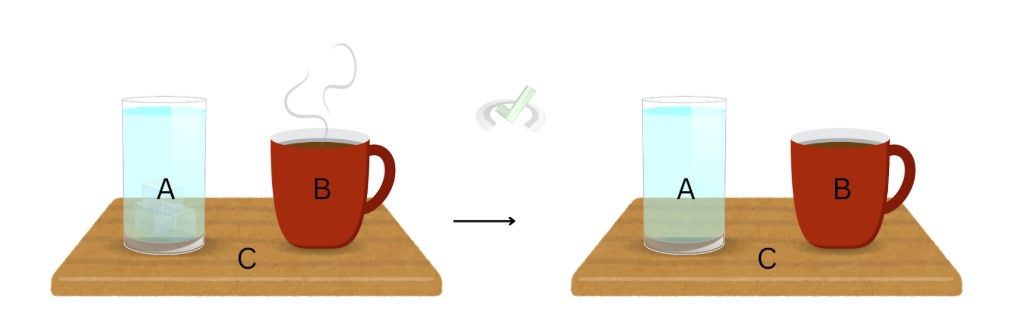
We can see thermal equilibrium in objects that have the same temperature. A glass of water will be in thermal equilibrium with a cup of a previously hot cup of coffee if the coffee is left out on the same table since heat will naturally radiate towards its surroundings, and some of this heat can naturally move towards the surface on which the cup sits. When the cup of coffee cools down until it reaches the same temperature as a glass of water, these two objects are in thermal equilibrium.
If we apply the Zeroth law to the glass of water and the cup of coffee, we can then say that after some time, when the surface or the table that has both the cup and the glass lose all the heat, it may have gotten from the cup, the three objects are in thermal equilibrium with each other.
If we look at things mathematically, given that A, B, and C refer to three different objects and that T is denoted as temperature, we can look at the Zeroth law as:
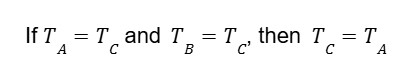
II. The First Law of Thermodynamics
“Energy can neither be created nor destroyed; it can only be changed from one form to another.”
-Albert Einstein
Although the line above is from Albert Einstein, scientists gradually developed the concept of the first law overtime before his time. The first law is also known as the law of conservation of energy. This law directly translates to its alias; it declares that energy in a system is conserved. Energy is not something that can be made or destroyed. When energy (or heat energy) is added to a system and consumed, it does not disappear–it simply transforms into a different form of energy.
When we use a heater inside a closed room, we need electricity to make it work. This electrical energy does not simply disappear when we turn on a heater; it transforms into heat energy. As heat dissipates into the room, everything inside the room warms up. The first law says that because heat energy does not disappear and is merely transformed into another form, the energy supplied by the electricity must be the same as the increase in thermal energy inside the room. While it cannot be the same form of energy as before, the energy consumed must theoretically be the same as no electrical energy was lost.
We mathematically define the first law as:
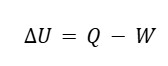
Where
- ΔU is the change in internal energy in the system
- Q is heat added to the system
- W does the system do the work
If we add heat while working on the system (+Q, +W), the internal energy increases, indicating a rise in energy because more heat enters as work consumes the energy. If we add heat as work is done by the system (+Q, -W), the internal energy still increases, which means that the system still gets more energy than it loses by doing work. When we remove heat while work is being done on the system (-Q, +W), the internal energy decreases and loses energy because it loses energy due to work more than it gains from heat. Finally, if we remove heat while work is being done by the system (-Q, -W), the internal energy also decreases as the system loses energy by doing more work than it gets through heat.
III. The Second Law of Thermodynamics
“The total change in entropy of a system plus its surroundings will always increase for a spontaneous process.”
Let’s first define what entropy is: we define entropy as a measure of disorder. The second law states that all systems tend to lean towards complete randomness. This may be a difficult concept to wrap our heads around, but the idea is this: everything wants disorder. Consider a block of ice left on a table. The molecules inside ice are ordered just as the molecules of any solid are—but as it melts, it turns into liquid,d and the molecules become more disordered. The ordered structure of the ice tends to change towards a more disordered structure.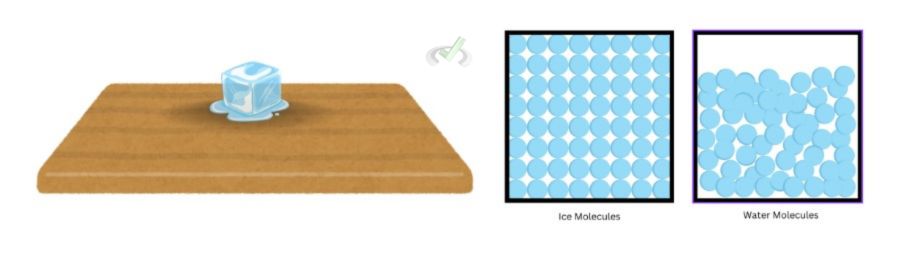
When we place a block of ice on a table, ice will naturally melt. All particles move; however, particles with more thermal energy tend to move a lot faster, whereas cold particles with less thermal energy tend to be stiff or rigid and only have movement through minute vibrations. The heat surrounding the ice naturally wants to spread more heat and cause more movement or disorder in its surroundings. By reaching thermal equilibrium, more disorder occurs in the system as more thermal energy or movement between particles occurse.
IV. The Third Law
“The entropy of a perfect crystal at absolute zero is exactly equal to zero.”
Crystals have an ordered structure, and unlike ice, they do not melt when left at room temperature. The third law discusses a perfect crystal at absolute zero or 0 Kelvin or -273.15 degrees Celsius. A perfect crystal is a theoretical crystal with a perfect shape, a constant arrangement of particles, and no missing atoms. Theoretically, the entropy of a perfect crystal will be at zero at the absolute zero, where there is minimal to no movement between particles. While the perfect crystal, let alone one at absolute zero, is highly unlikely, the third law explains entropy more beautifully.
Absolute zero is a theoretical but important reference in our temperature scales. It sets the lowest possible temperature at which particles are not in complete entropy or randomness. We can assume or use this as a benchmark for how matter behaves at extremely low temperatures. Because the second law talks about how everything tends to move towards a state of more chaos or disorder, the third law explains how difficult the lack of disorder is and how objects naturally avoid being in a state where no entropy exists.V. Conclusion
The laws of thermodynamics perfectly show the principles that explain everything that we cannot see, unlike how we can say that when we apply a huge force and cause an object to move, we cannot see how things work on a microscopic scale. These laws explain the behavior of energy and how it changes. These laws can provide the basic concept of how energy is transferred and all the other biochemical reactions in our bodies.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
