When baking a cake, cookies, or brownies, one thing that’ll probably be included in all the recipes is sugar, as it’s a basic ingredient to make these dishes sweet! Similarly, redox reactions are a staple in all chemistry subclasses including general, organic, and biochemistry!
Redox reactions are such a fundamental reaction that you can argue that organic chemistry is based around redox reactions (of course, a generality). However, general chemistry also puts a great emphasis on redox reactions!
After going through this chapter overview, you’ll get a better understanding of redox reactions in the context of general chemistry. Let’s get started!
Redox Reactions on the MCAT: What You Need to Know
Topics on general chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
Luckily, redox reactions in general chemistry is not as heavily tested as other general chemistry topics! Take this time instead to establish the fundamentals of redox reactions so that you can apply them when studying redox reactions in organic chemistry and biochemistry.
Introductory general chemistry accounts for 30% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Redox Reactions
When put into the context of general chemistry, you’ll see that there’s a more “number” and “math” focus. You’ll get a better idea of this in the upcoming sections as these deal with assigning oxidation numbers and balancing half reactions.
Contrast this to redox reactions when put into the context of organic chemistry! In organic chemistry, we took a more general approach as focused more on why attaching bonds to certain atoms either oxidizes or reduces a molecule.
1. Main Definitions of Oxidation and Reduction Reactions
Differentiating between oxidation and reduction is fairly simple: oxidation involves the loss of electrons while reduction involves the gain of electrons. You may have heard of the popular mnemonic “OIL-RIG” which refers to “Oxidation is Losing (Electron); Reduction is Gaining (Electron)”.
2 other important terms to differentiate are oxidizing and reducing reagents. The oxidizing reagent is the species (or better yet the reactant) that gets reduced while the reducing reagent is the species/reactant that gets oxidized.
Be careful with these terms as their name is the opposite of what happens to them! To get a better understanding let’s give an example! Shown below is a common redox reaction which occurs in anaerobic glycolysis: lactate fermentation.
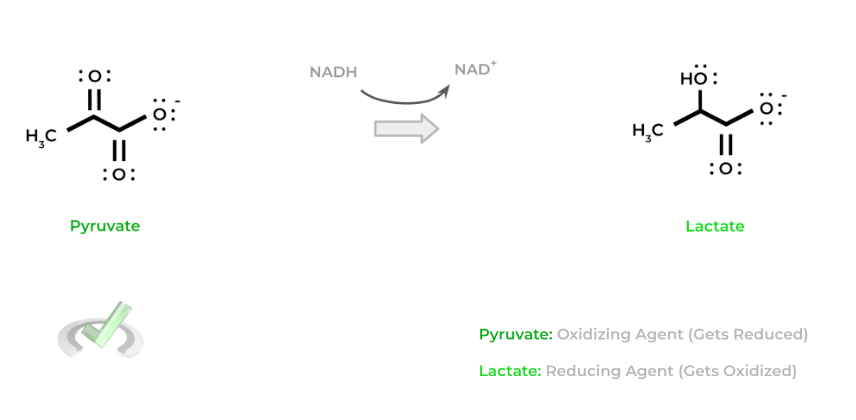
In this case, pyruvate gets reduced to lactate and thus acts as the oxidizing agent. Likewise, NADH gets oxidized back to NAD+ and acts as the reducing agent. Another way to think of it is that pyruvate oxidizes NADH to NAD+ which is why it's the oxidizing agent (and vice versa).
Full Study Notes : Basic definitions and principles of oxidation and reduction reactions
For more in-depth content review on basic definitions and principles of oxidation and reduction reactions, check out these detailed lesson notes created by top MCAT scorers.
2. Assigning Oxidation Numbers for Chemical Species
In stoichiometry, we’re usually worried about making sure we have the right amount of atoms on each side to fulfill the conservation of mass. However, we also must make sure the charge is the same on both sides of the redox equation in order to fulfill the conservation of charge as well!
In order to keep track of the charges of the atoms, we can assign them oxidation numbers. A great way to think about oxidation number is as the hypothetical charge of an atom in a molecule if the molecule was connected by ionic bonds.
Let’s use HCl as an example! Although HCl is connected by a covalent bond, let’s pretend that it’s connected via an ionic bond when assigning oxidation numbers. In this case, hydrogen and chloride have oxidation numbers of +1 and -1, respectively.
There are 8 main rules (with accompanying exceptions) that must be followed when assigning oxidation numbers as well, listed below:.
1. Free elements/neutral species always have an oxidation number of +0. These primarily include molecules composed of only one element — some great examples are the diatomic gas molecules like N2, F2, I2, etc.
2. Monatomic ions, such as Na+ and Mg+2, have the same oxidation number as their charge! For example, Mg+2 has an oxidation number of +2.
3. Group IA elements (Na, K, etc.) always have an oxidation number of +1.
4. Group IIA elements (Ca, Mg, etc.) always have an oxidation number of +2.
5. Hydrogen atoms will usually have an oxidation number of +1.
**Exception 1:
Except when bonded to a less electronegative atom. Look at the example below!
6. Oxygen atoms will usually have an oxidation number of -2.
**Exception 1:
Except when they’re included in peroxides – in this case, they have an oxidation number of -1. Look at the example below!
**Exception 2:
Except when bonded to a more electronegative atom – in this case, they have an oxidation number of +2. Look at the example below!7. Group VIIA (Cl, Br, etc.) elements will usually have an oxidation number of -1.
**Exception 1:
Except when bonded to a more electronegative atom – in this case, they have an oxidation number of +1. Look at the example below!
8. For a neutral compound, the sum of the oxidation numbers for each atom in the compound must equal +0. Likewise, for a polyatomic ion, the sum of the oxidation numbers for each atom in the compound must equal the ion charge.
**Important Tip!
Don’t forget to also account for the number of atoms of a particular element when summing the oxidation numbers!
In H2O, for example, hydrogen has an oxidation number of +1. However, because there are 2 hydrogen atoms, they both sum up to a +2 oxidation number.
Full Study Notes : Rules of assigning oxidation numbers for atoms in compounds
For more in-depth content review on rules of assigning oxidation numbers for atoms in compounds, check out these detailed lesson notes created by top MCAT scorers.
3. Oxidation Number v.s. Formal Charge
An important distinction must be made between oxidation number and formal charge, as both are ways to assign hypothetical charges to chemical species. The main distinction lies in how the electrons are assumed to be distributed.
When assigning oxidation numbers, we assume that the electrons between 2 atoms are distributed unequally, where all the electrons are given to the more electronegative atom. Conversely, assigning formal charges assumes that the electrons are shared equally between the atoms. Look at the example below!
Another main difference between the 2 is when we utilize one system or the other. Oxidation numbers are useful as we can keep track of oxidation states in redox reactions! Formal charges are primarily used for determining which Lewis structure of a molecule is most appropriate (as multiple structures can be drawn).
One final note in regards to oxidation number and the formal charge is that no one system is entirely correct. Instead of electrons being given entirely to the more electronegative atom or electrons being shared evenly, it’s really somewhere in between, realistically.Full Study Notes : How to calculate the formal charge
For more in-depth content review on how to calculate the formal charge, check out these detailed lesson notes created by top MCAT scorers.
4. Stoichiometric Balancing of Redox Reactions
As mentioned before, we should also be wary when balancing redox reactions as we have to consider both the balancing of the atoms and charges of the reaction! Similar to assigning oxidation numbers, we also have a certain step-by-step process to follow! In this case, we have 5 main steps to follow:

1. Split the reaction into 2 half-reactions, as shown below!
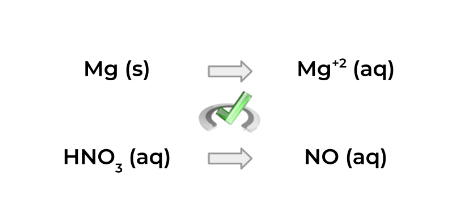
2. This step will actually have three subparts (sorry!)
a. First, balance all the atoms EXCEPT for hydrogen (H) and oxygen (O). In this case, all the other atoms aside from H and O are already balanced.
b. Next, balance the O atoms by adding water (H2O) to the appropriate side and putting the proper coefficient. In this case, we just worry about the bottom half-reaction. Check how it’s done below!

c. Now, balance the H atoms by adding a proton (H+). However, you must identify first whether the reaction is occurring in an acidic or basic solution as this will be important for later.

If the reaction is occurring in an acidic solution, you can be finished here. However, if the reaction occurs in a basic solution, you must also add hydroxide ions (OH-) on both sides of the equation in order to neutralize the H+ protons.
The H+ will then combine with the OH- ions to form water molecules. We can then simplify the equation with any excess H2O.
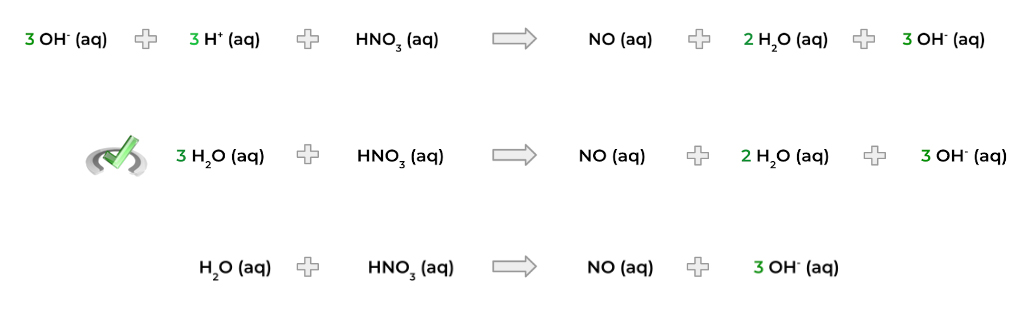
3. Add the appropriate amount of electrons to each half reaction to balance out the charge! In the example below – and for the rest of the overview – we’ll assume acidic conditions.
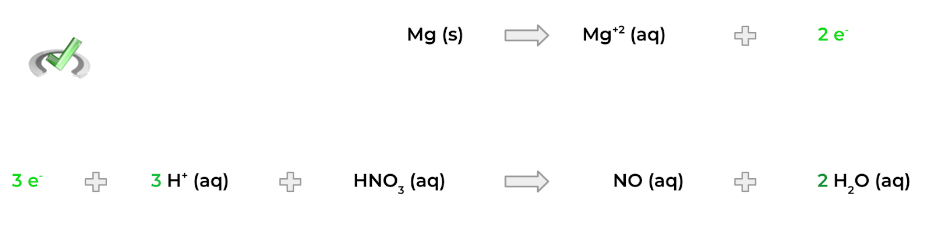
4. Now, multiply each half reaction by the proper factor so that there’s an equal number of electrons! For example, we can get 6 electrons by multiplying the top half reaction by 3 and the bottom half reaction by 2!
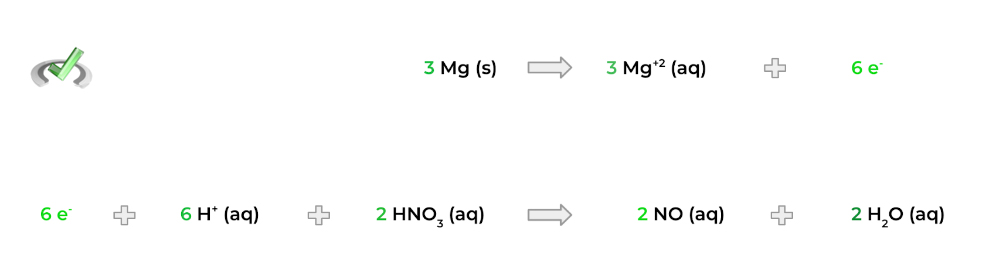
5. Finally, add both the half reactions to get one total reaction! Note that the electrons will cancel each other out and that the overall charge on both sides of the equation will be equal.

Full Study Notes : Redox reaction in a basic solution
For more in-depth content review on redox reaction in a basic solution, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about carboxylic acids and their derivatives in organic chemistry!
Term | Definition |
|---|---|
Oxidation | Occurs when a chemical species loses electrons |
Reduction | Occurs when a chemical species gains electrons |
Oxidizing Agent | Term which refers to the chemical species in a redox reaction which gets reduced |
Reducing Agent | Term which refers to the chemical species in a redox reaction which gets oxidized |
Oxidation Number | One way to assign hypothetical charges on molecules with the assumption that the electrons are distributed UNEQUALLY between atoms; Most used to keep track of charges in redox reactions |
Formal Charge | One way to assign hypothetical charges on molecules with the assumption that the electrons are distributed EQUALLY between atoms; Mostly used to determine the most appropriate Lewis Dot structure |
Additional FAQs - Redox Reactions in General Chemistry on the MCAT
How Do You Remember Oxidizing and Reducing Agents? – MCAT
The oxidizing agent is the species in the redox reaction that actually gets reduced, while the reducing agent is the species that gets oxidized.
What are the 5 Redox Reactions? – MCAT
These include combination/synthesis, decomposition, single substitution, double substitution, and combustion reactions!
How Do You Calculate Oxidation Number? – MCAT
Additional Reading: General Chemistry MCAT Topics:
- Atomic Structure on the MCAT
- Periodic Table on the MCAT
- Bonding and Chemical Reactions on the MCAT
- Chemical Kinetics on the MCAT
- Electrochemistry on the MCAT
- Equilibrium on the MCAT
- Solutions on the MCAT
- Stoichiometry on the MCAT
- The Gas Phase on the MCAT
- Thermochemistry on the MCAT
- Acids and Bases on the MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot