I. What is Amino Acid Structure?
If we had to make an NBA analogy to MCAT content, amino acids are basically the Michael Jordan when it comes to MCAT study content (Lebron too, but that’s debatable & probably best left to the pros – trust me this hurts to say as a Lebron fan).
Hopefully, by this analogy, you can see the great importance of amino acids during MCAT study. They are so important that we (and maybe others have told you as well!) here at MCAT Mastery highly recommend studying amino acids as the first topic to study in your MCAT prep!
You’ll without a doubt encounter questions on the MCAT in both the Phys/Chem & Biochem/Bio Section that will in some way or form test you about amino acids. This is why it is so important to know the amino acids like the back of your hand, including their names, structures, abbreviations, etc.
II. Fundamentals in Amino Acid Structure
There are 20 proteinogenic amino acids that are present in prokaryotes & eukaryotes; proteinogenic is really just a fancy term meaning that they are used as the building blocks to make proteins. Let’s first take a look at their backbone before getting into the different categorizations!
A. Backbone Structure of Amino Acids
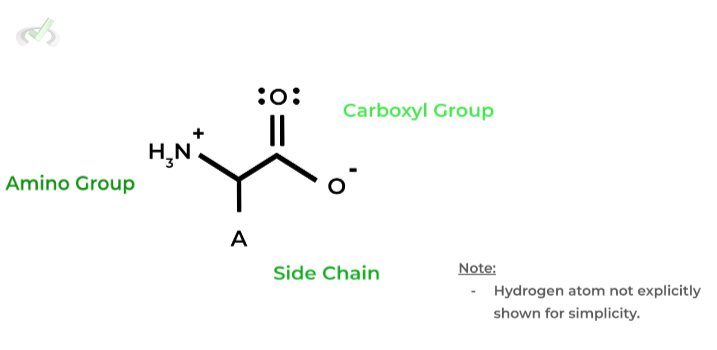
All amino acids will have all of the following: 1) an amino group (NH3+), 2) a carboxyl group (COO-), 3) a side chain, and 4) a hydrogen atom. All of these functional groups are connected to a central carbon termed the 𝛼-carbon.
The amino and carboxyl group will be important when discussing how peptide bonds are formed between amino acids to generate the protein polypeptides!
The side chain (depicted as “A”) is a chemical group unique to each amino acid and will determine the amino acid’s characteristics!
Backbone Amino Acid Structure
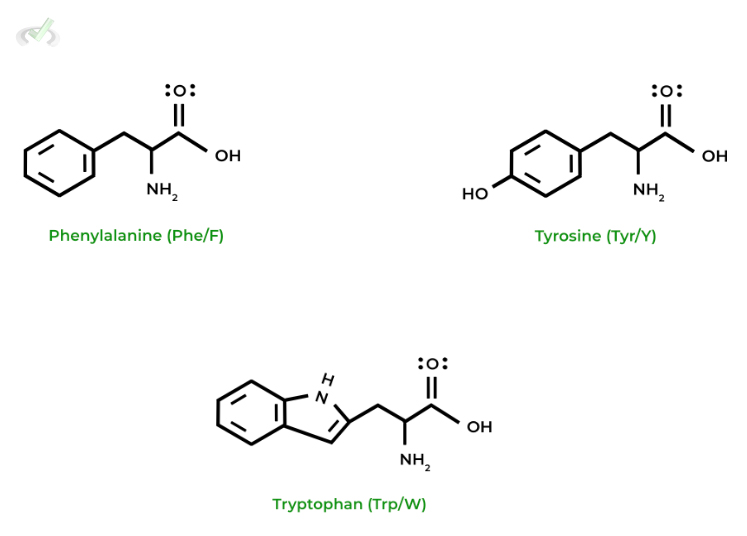
Tyrosine can be overall hydrophobic or hydrophilic, depending on the environment (e.g more hydrophilic in aqueous solution). The aromatic ring has nonpolar properties while -OH has polar properties and an ability to form hydrogen bonds. Please make sure to consider the context from passages or question stems!
B. The Proteinogenic Amino Acids
If we had to pick one topic to commit to memory, we would without a doubt recommend memorizing these amino acids in all aspects of names, structures, characteristics, letter abbreviations, etc.
Questions assessing your ability to recognize any of these aspects of amino acids will undoubtedly come up on the MCAT and we want to make sure you’re prepared to tackle these questions.
Though memorizing these may be a daunting task, perhaps first categorizing and chunking certain amino acids will be a useful approach for memorization. We categorize amino acids by similar characteristics which are a result of their side chains!
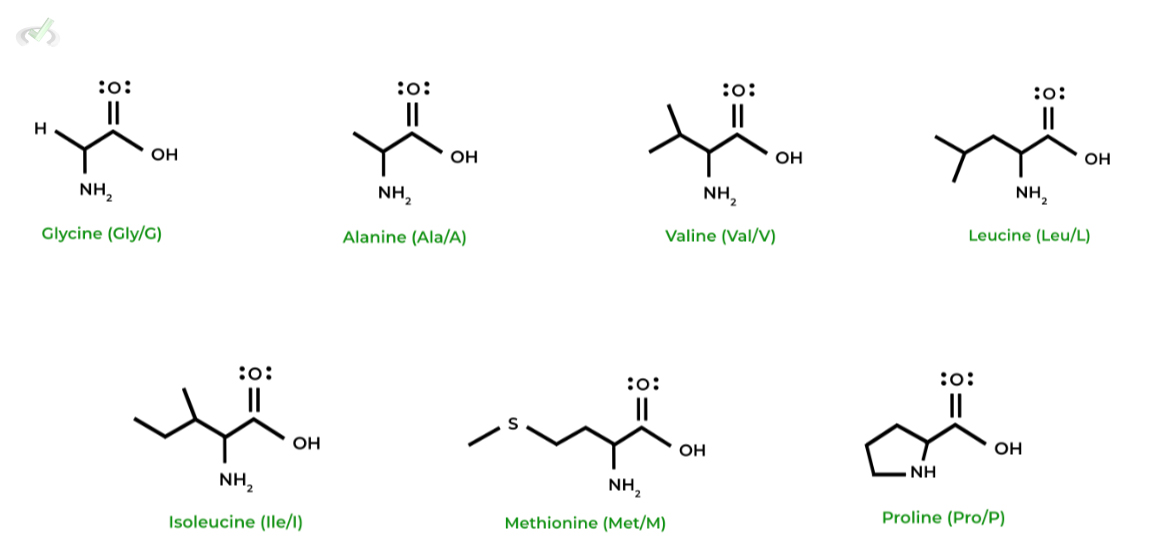
I. Nonpolar, Aliphatic Amino Acids
These amino acids are very hydrophobic due to their nonpolar nature. Much of this has to do with their aliphatic characteristic, which is a term that refers to open hydrocarbon chains!
II. Nonpolar, Aromatic Amino Acids
Similarly, these amino acids are also hydrophobic as they’re nonpolar; however, this is due to the presence of an aromatic ring within their side chains!
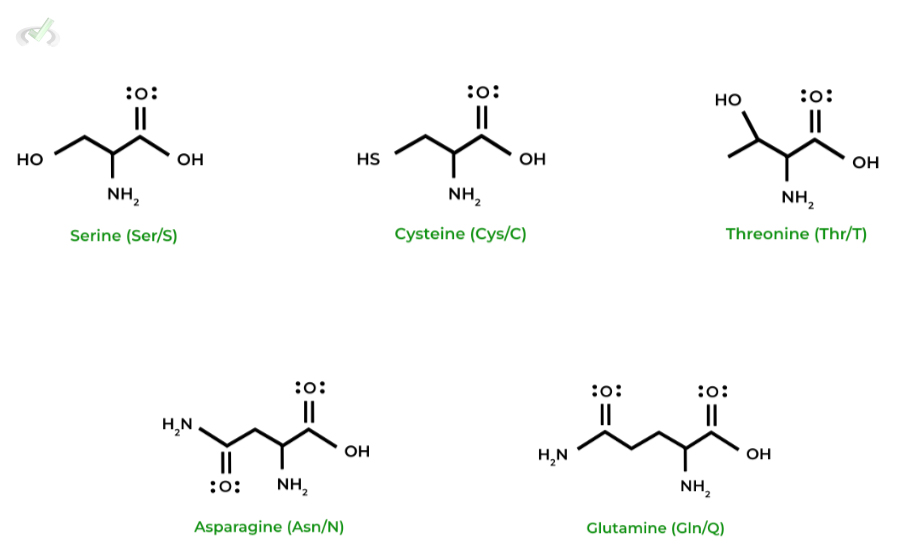
III. Polar Amino Acids
In contrast to the previous 2 categories, these amino acids are hydrophilic due to their polar nature, having the ability to form favorable dipole-dipole and hydrogen bonds interactions!
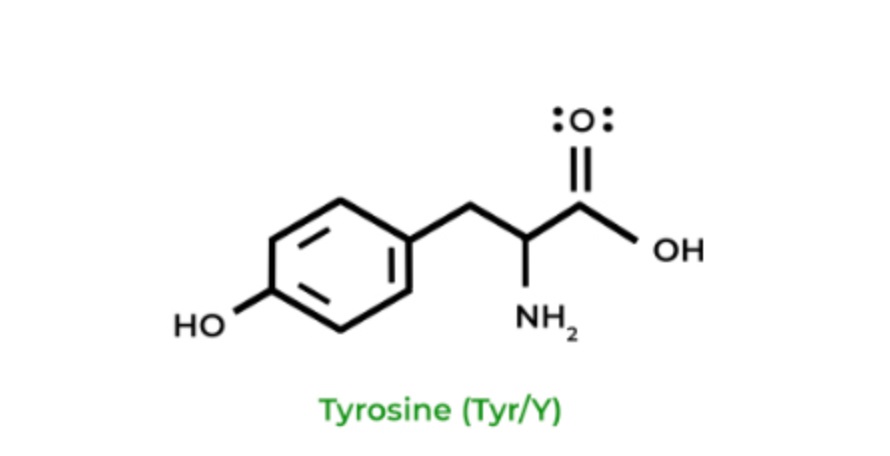
Tyrosine can be overall hydrophobic or hydrophilic, depending on the environment (e.g more hydrophilic in aqueous solution). The aromatic ring has nonpolar properties while -OH has polar properties and an ability to form hydrogen bonds. Please make sure to consider the context from passages or question stems!
IV. Acidic (Negatively Charged) Amino Acids
As indicated by their names, these amino acids have an overall NEGATIVE molecular charge at physiological pH. This is due to the relatively low pKas of the functional groups present on the side chains!
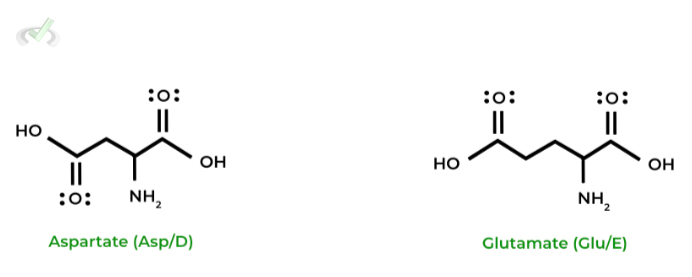
Notice also how aspartate and glutamate have essentially the same structure as asparagine and glutamine, respectively; however, they differ only in their functional groups!
As opposed to the amide groups present on asparagine and glutamine, aspartate and glutamate have carboxyl groups!
When working on memorizing the structures, try and find these similarities to aid in your memorization!
V. Basic (Positively Charged) Amino Acids
As opposed to their negative charged counterparts, these amino acids have an overall POSITIVE molecular charge due to the high pKas of the functional groups present on the side chain!
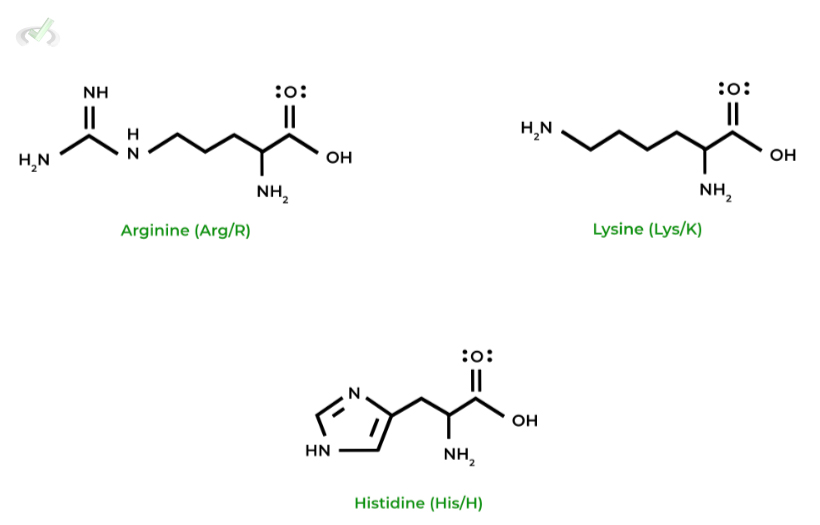
It is important again that we stress to you all to memorize all the amino acids in terms of their names, structures, characteristics, letter abbreviations, etc.
Although organizing them way above may help, we definitely understand it can be overwhelming to memorize all this stuff. (We definitely know from personal experience!)
Take a look at our “TIps on Memorizing Amino Acids” article which goes over some tips & strategies we’ve come up with to hopefully help you all in this task!
III. Bridge/Overlap
We talked about how pKas of the functional groups are important in conferring the charge on acidic and basic amino acids. Let’s quickly review pKas and their role in amino acid charge!
I. pKas in Amino Acids
Recall that pKas are a numerical value that determines a functional group's protonation state in the context of pH!
When the pH of a solution is LESS than the pKa of a functional group, the functional group will be PROTONATED. When the pH of a solution is GREATER than the pKa of the functional group, the functional group will be DEPROTONATED.
Overall amino acid charge are affected by the sum of the charges of the functional groups, including the amino group, carboxyl group, and side chain. The pKas of the amino group and carboxyl group are around 2 & 9 respectively, with the pKa of the side chain being unique for each side chain.
Let’s look at aspartate as an example: since it also has a carboxyl group on the side chain, it also has a similar pKa, about 3.9.
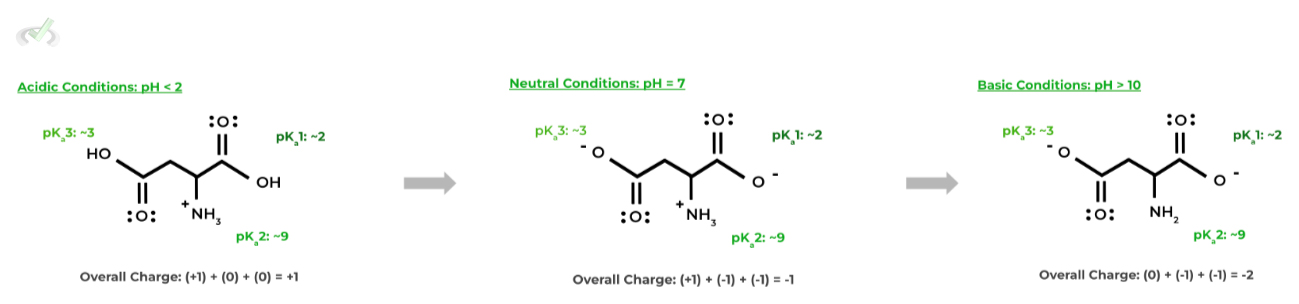
Hopefully this gives a better picture of why aspartate is a negatively charged amino acid: in neutral conditions, the pH is greater than the pKas of both carboxyl groups, deprotonating them resulting in 2 negatively charged carboxylate groups.
The negatively charged carboxylate groups thus contribute to the overall negative charge of aspartate in neutral, physiological conditions.
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Backbone Structure of Amino Acids
All amino acids will have: 1) an amino group (NH3+), 2) a carboxyl group (COO-), 3) a side chain, and 4) a hydrogen atom, all connected to an 𝛼-carbon.
The amino and carboxyl group are important in the formation of peptide bonds while the side chain's functional groups will give each amino acid a certain characteristic!B. The Proteinogenic Amino Acids
Amino acids can be grouped based on similar characteristics which are conferred by the side chain as mentioned earlier! Make sure to memorize the amino acids in terms of names, structures, characteristics, letter abbreviations, etc.
I. Nonpolar, Aliphatic Amino Acids
These are hydrophobic amino acids due to their nonpolar, aliphatic nature, which is conferred by the presence of open hydrocarbon chains!
II. Nonpolar, Aromatic, Amino Acids
Similarly, these amino acids are hydrophobic due to their nonpolar nature; however, in this case, it’s due to the presence of aromatic hydrocarbon rings.
III. Polar Amino Acids
Contrarily, these amino acids are hydrophilic due to their polar nature, which allows them to form favorable dipole-dipole and hydrogen bonds interactions!
IV. Acidic (Negatively Charged) Amino Acids
As indicated by the name, these amino acids have an overall NEGATIVE molecular charge at physiological pH due to the relatively low pKas of the side chain’s functional groups.
V. Basic (Positively Charged) Amino Acids
Contrarily, these amino acids have an overall POSITIVE molecular charge as physiological pH due to the relatively high pKas of the side chain’s functional group.
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
Aspartate is a negatively charged amino acid because it contains an extra _________ group while lysine is a positively charged amino acid because it contains an extra _________ group, respectively.
A. Carboxyl, Carboxyl
B. Amino, Amino
C. Carboxyl, Amino
D. Amino, Carboxyl
Ans. C
When looking at the structures of aspartate & lysine, it can be seen that aspartate does have an extra carboxyl group while lysine does have an extra lysine group.
This should make sense, as in physiological pH, the carboxyl group is deprotonated and takes on a negative charge (-1) while the amino group is still protonated and takes on a positive charge (+1).
Hence, this is why aspartate is a negatively charged amino acid while lysine is a positively charged amino acid.
Sample Practice Question 2
Which of the following statements is false?
A. Asparagine and Glutamine are most likely present on the transmembrane domain of a protein
B. Glycine is the smallest amino acid.
C. Serine & Threonine are most likely present on the surface of a cytosolic protein.
D. At physiological pH, one only of the functional groups is protonated in valine.
Ans. A.
Because asparagine and glutamine are polar amino acids, these would have unfavorable interactions within the inner membrane as it’s hydrophobic.
However, all the other statements are true:
Glycine is the smallest amino acid because its side chain is just another hydrogen atom.
Serine & Threonine are polar amino acids that should be expected to be on the surface of a cytosolic protein, as it will have favorable interactions with water molecules in the cytoplasm.
At physiological pH (~7.4), only the amino group of valine will be protonated as the carboxyl group is already deprotonated.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot