I. What are Other Forms of Enzyme Inhibition?
Hi again & welcome back, assuming again you’re coming from our other enzyme inhibition study article! You can probably see by the amount of enzyme articles how important this topic is to the MCAT, from enzyme structure all the way to the different ways enzyme function can be disrupted. This article can be thought of as an extension of the enzyme reversible inhibition article!
While the enzyme puts a high amount of focus on reversible enzyme inhibition through drugs, there are also several other ways that enzymes can be inhibit from their function, from the type of environment they’re in all the way to irreversible inhibition where essentially the enzyme function is eliminated completely and cannot be restored (unlike reversible inhibition).
When studying and reviewing this article, keep an eye for what other connections and bridges can be made to other topics in MCAT review!
II. Content Review
The whole reason for regulating enzyme functioning is to maintain homeostasis on a smaller level so as to further this homeostasis on a whole body, systemic level! We’ll primarily cover 3 main ways enzymes can be regulated aside from reversible inhibition:
A. Environmental Conditions
Just like all proteins, changes in the environmental conditions that the enzyme is surrounded in can cause changes in enzyme structure and function! Check out our “Protein Structure” Study Article for a review of these changes!
While we already covered what different environmental changes can cause alteration in enzyme structure and function will primarily focus on how pH can alter enzyme function!
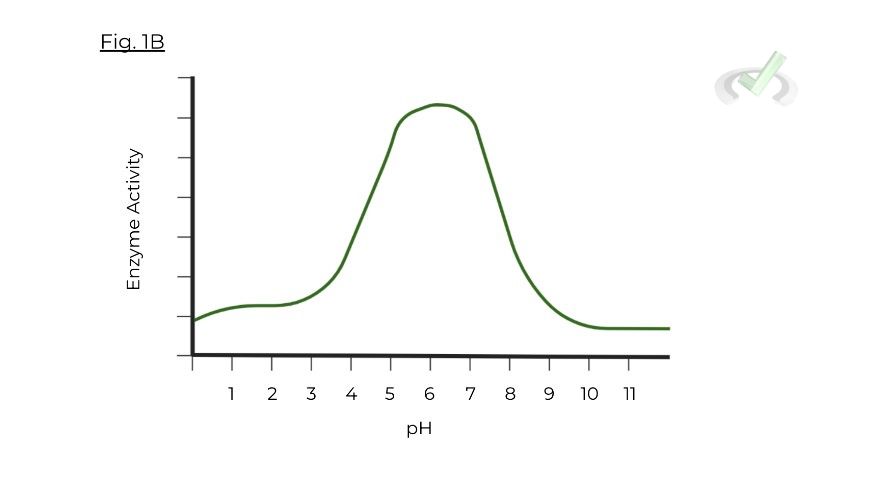
Enzymes are unique in the sense that there is not 1 optimal pH range that enzymes must be in, but a whole range!
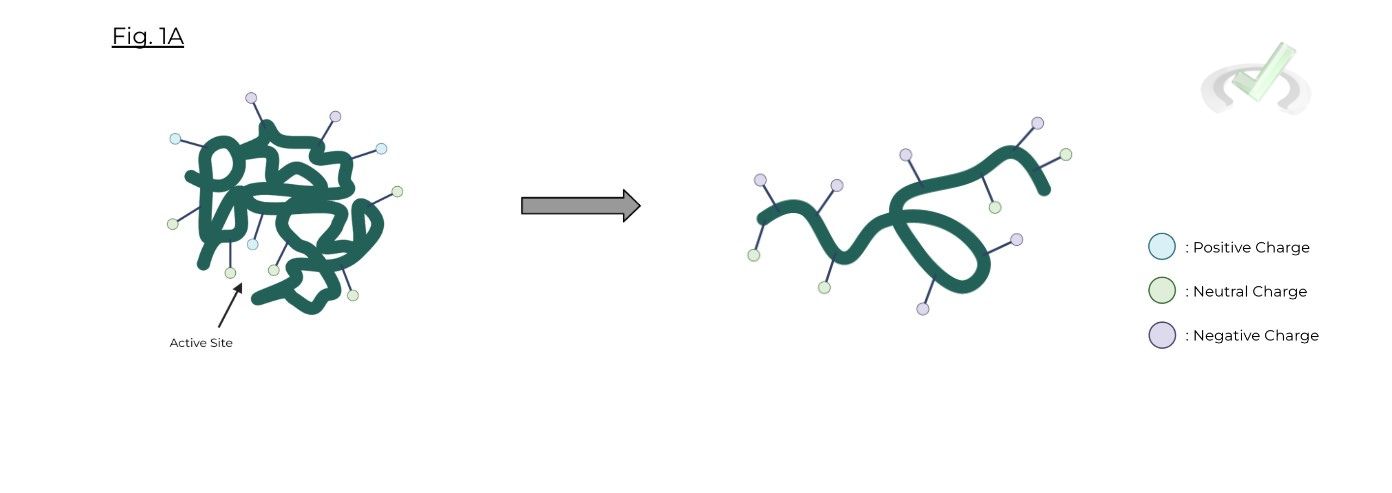
Changes in an enzyme’s optimal pH range are particularly damaging to enzyme function because it can change the ionization state of both the 1) active site and the enzyme’s 2) tertiary structure!
Furthermore, take a look at the following graph below. Based on the axes, you can see that for this particular enzyme, there is an optimal pH range where the enzyme can function with high activity.
B. Irreversible Inhibition
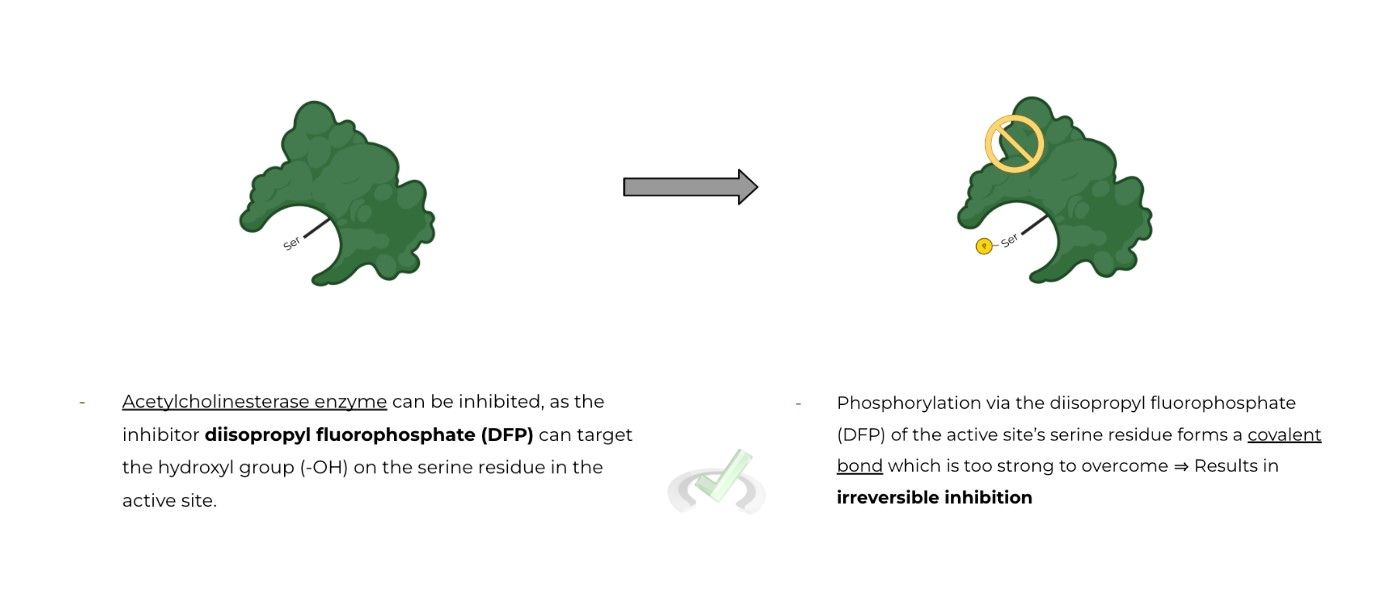
Irreversible inhibition is exactly what it sounds like! Unlike reversible inhibitors, irreversible inhibitors cause a PERMANENT change in the enzyme’s structure & function to a point where it CANNOT be recovered
Most of the time, irreversible inhibitors form covalent bonds with the enzyme in order to result in a permanent change to the enzyme’s function. This is opposed to reversible inhibitors which mostly form non-covalent bonds with the enzyme.
Because covalent bonds involve the sharing of electrons, they are generally stronger bonds than noncovalent; hence why it's difficult to overcome the effects of irreversible inhibition.
C. Zymogens
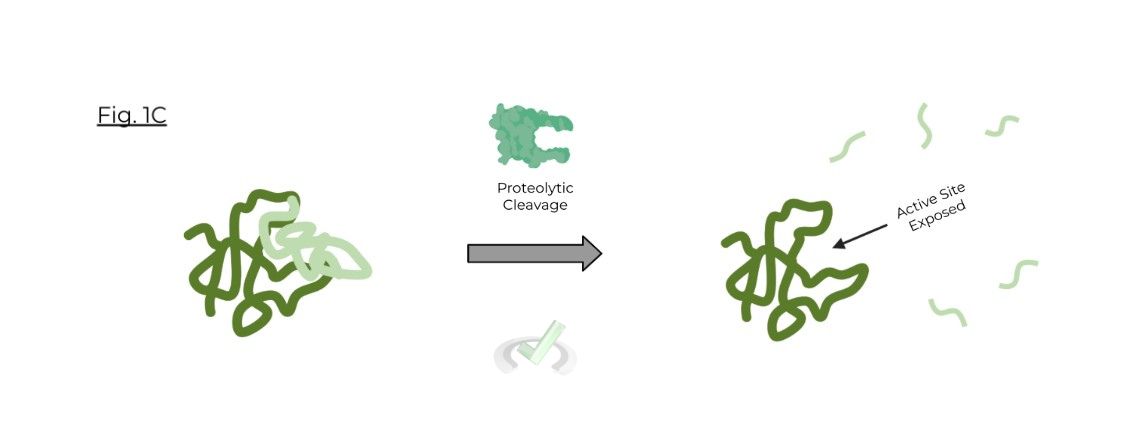
Zymogens are inactive precursor forms of enzymes that require a biochemical change in order to become a functional enzyme; just think of them as enzymes which need a couple more steps to become functional.
These changes can come in a variety of forms, but are most likely due to proteolytic cleavage by another enzyme or a change in environmental pH in order to expose the active site
As you’ll see in the bridge/overlap and in our other study articles, many zymogens will usually have either the prefix “-pro” or the suffix “-gen”.
Think of zymogens like an untrimmed bonsai tree! They’re still a tree but it’s not until the leaves are trimmed that the tree actually becomes a bonsai tree, similar to zymogens which need proteolytic cleavage.
III. Bridge/Overlap
Digestive enzymes are a prime example of how pH and zymogens work to regulate enzyme function. Figure 1B actually shows an accurate modeling of the optimal pH range of a digestive enzyme in the small intestine! Let’s take a look at these 2 examples to put a real biological perspective.
Stomach
Pepsinogen is a zymogen which is released by the chief cells of the stomach ⇒ Will only become activated to “pepsin” under acidic conditions to prevent proteolytic degradation of the stomach!
Upon ingestion of food, the parietal cells of the stomach will release hydrochloric acid (HCl), which decreases the pH of the stomach ⇒ Pepsinogen undergoes conformational change to expose active site, becoming activated
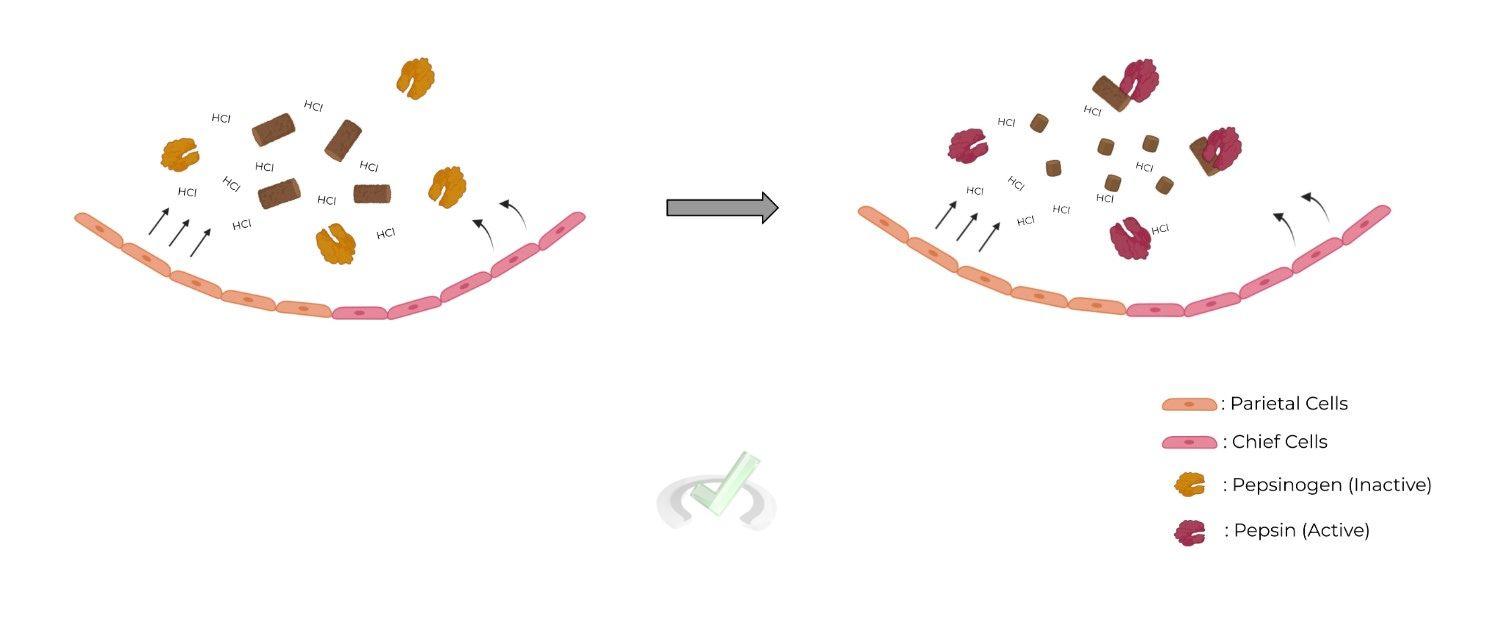
Small Intestine
The small intestine is a little more complex, as it contains a system of proteolytic enzymes which cleave & activate downstream zymogens.
Begins with enteropeptidase cleaving trypsinogen to active trypsin. Trypsin then cleaves chymotrypsinogen & procarboxypeptidase A/B to active chymotrypsin and carboxypeptidase A/B -- enteropeptidase can also cleave procarboxydase to small extent!
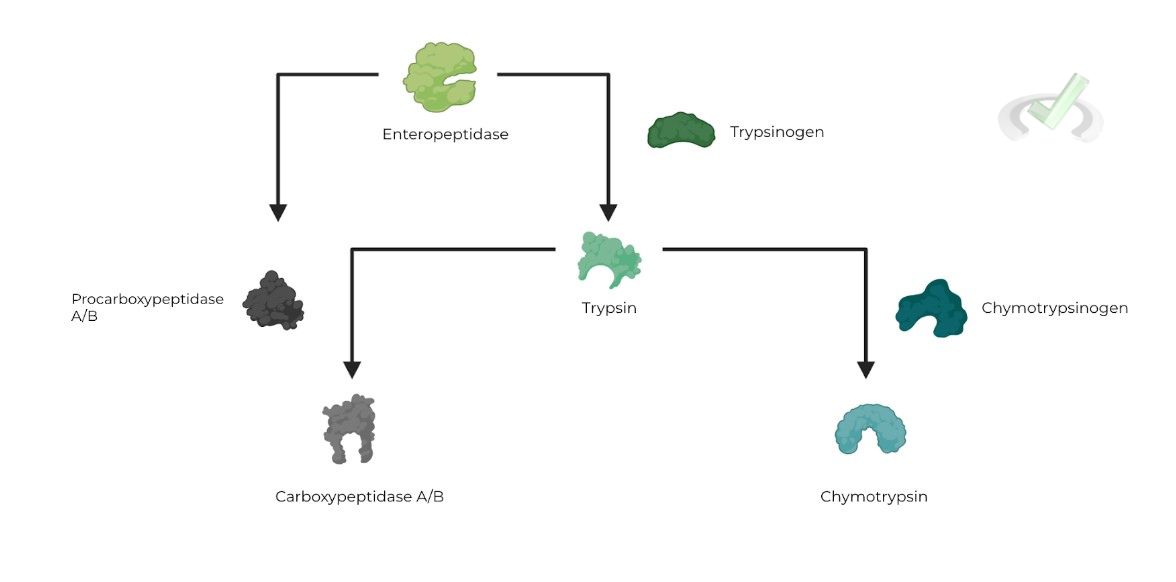
Just like pepsinogen, enzymes that function in the small intestine also function in an optimal pH range; for them, however, it's much more basic!
Secretin and Cholecystokinin (CCK) are hormones that allow for the release of substances which increase the pH of the small intestine. Again, take a look back to figure 1B as an example of an optimal pH range of a digestive enzyme in the small intestine.
Notice also how hormones play a role in enzyme regulation!!
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
Enzyme Inhibition: Other Forms
Though the most likely form of enzyme inhibition you’ll encounter will be reversible inhibition, there are several other ways enzymes can be inhibited! Those covered in the article include:
I. Environmental Conditions:
Just like we talked about in our “Protein Structure” study article, several environmental conditions can affect how the enzyme functions, including pH, salinity, and temperature.
Changes in pH can lead to changes in the protonation & charge of the active site and the tertiary structure of the enzyme!
II. Irreversible Inhibition:
Will affect the enzyme where inhibition is impossible (or very hard) to overcome, resulting in a complete loss of function of the enzyme which cannot be recovered.
Often will form a covalent bond with the enzyme (as opposed to reversible inhibitors, which usually form non covalent bonds).
III. Zymogens:
Termed as inactive, precursor enzymes which requires a biochemical change (such as proteolytic cleavage or environmental pH) in order to expose the active site
Will often have the prefix “-pro” or suffix “-gen”!
The digestive enzymes are a prime example of the above mentioned enzyme inhibition methods!
Pepsinogen, an zymogen present within the stomach, will only become activated under acidic conditions
The enzymes which function in the small intestine require a more basic pH in order to function, as well as engaging in a complex cascade of proteolytic cleavage in order to become activated!
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
How would the via value of vmax change upon administration of an irreversible inhibitor?
A. Increases
B. Decreases
C. Remains Constant
D. Need More Information
Ans. B
Recall your understanding of why other types of inhibitors cause a change in vmax. Irreversible inhibitors affect the enzyme in a way where inhibition is too hard to overcome and basically renders the enzyme permanently inactive.
As such, when the irreversible inhibitor is administered, the amount of available functional enzymes present DECREASES. According to the maximum enzyme velocity equation, a decrease in the total [E] should lead to a decrease in vmax.
Sample Practice Question 2
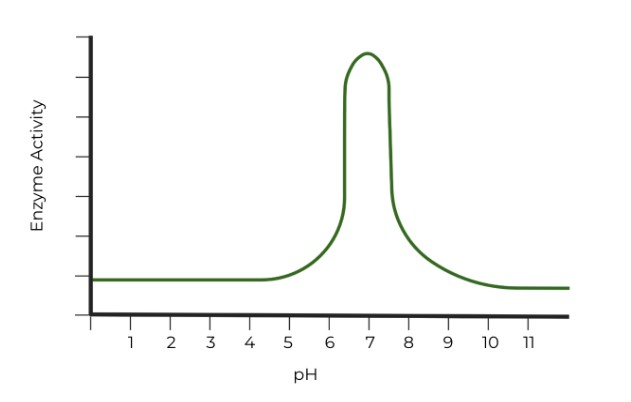
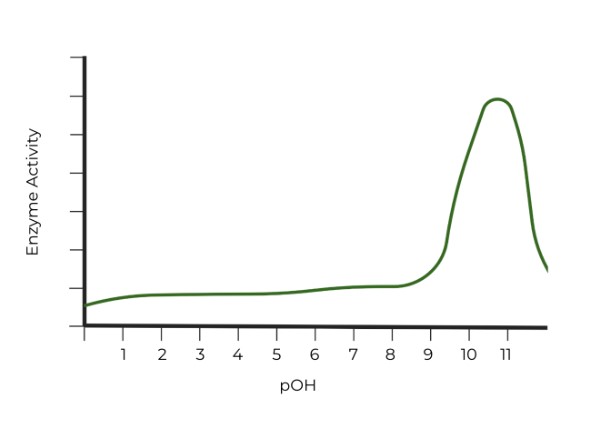
Which of the following graphs properly represents the optimal pH range of pepsin? (Hint: Watch the axes!)
A.
B.
C.
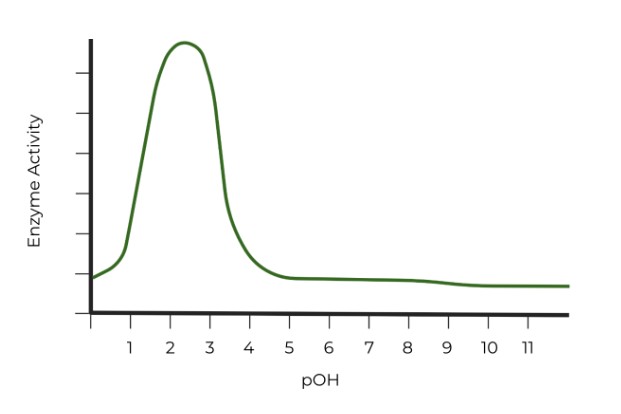
D.
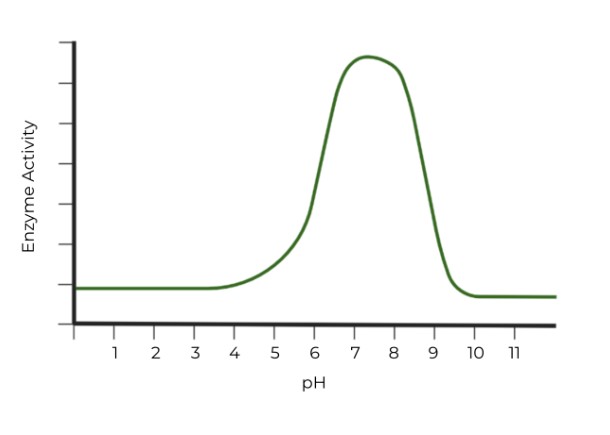
Ans. B
As noted in the hint given above, pay very close attention to the axes! Although we’re covering enzymes in this study note section, it’s important to recognize these various overlaps, as this is how the MCAT will approach questions!
Pepsin is a type of stomach digestive enzyme which functions optimally at low (acidic) pH.
However, pay close attention to the axes titles! It may be easy to say that C. has the correct graph, but the x-axis in pOH units. Remember, the sum of pOH and pH should equal 14, so the pH values which give the enzyme the highest activity in graph C are around 11 - 13, very basic pH values!.
In graph B, the pOH values which give the enzyme the highest activity are around 10 - 12, which means that the corresponding pH values would be around 2-4, very acidic pH values. !







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot