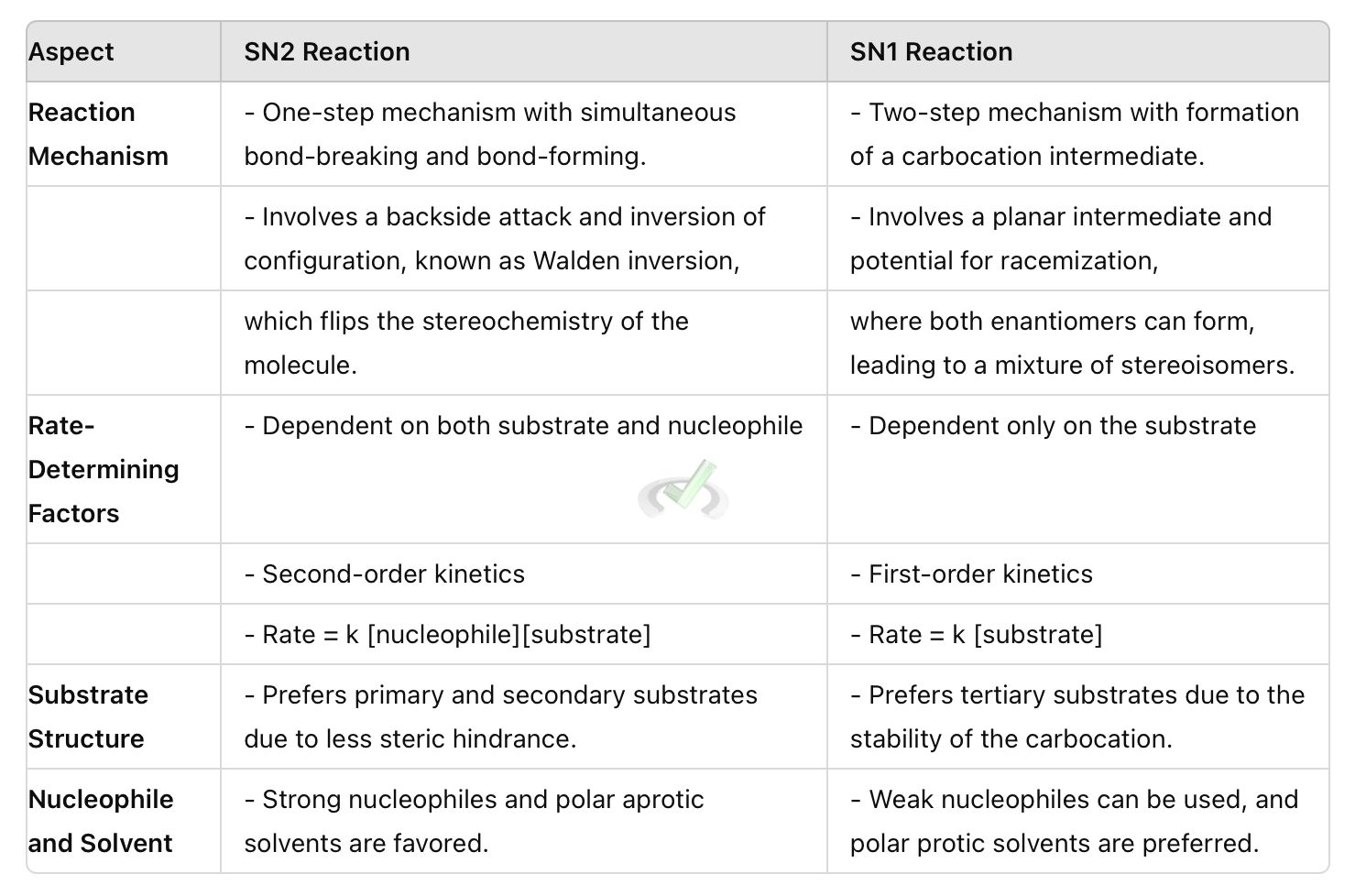
Understanding reaction mechanisms is crucial in organic chemistry. Two important types of nucleophilic substitution reactions are SN2 and SN1. These reactions involve replacing a leaving group with a nucleophile. The mechanism and rate of these reactions depend on various factors.
I. Basics of SN2 and SN1 Reactions
SN2 Reactions
The term SN2 stands for Substitution Nucleophilic Bimolecular. In this reaction, the nucleophile attacks the substrate at the same time as the leaving group leaves. This process happens in one step, without any intermediates.
Step 1: Nucleophilic Attack
The nucleophile (Nu⁻) attacks the carbon atom bonded to the leaving group (R-LG) from the opposite side of the leaving group. This backside attack leads to an inversion of configuration at the carbon center.
Step 2: Leaving Group Departure
As the nucleophile forms a bond with the carbon, the leaving group departs simultaneously.

Example:
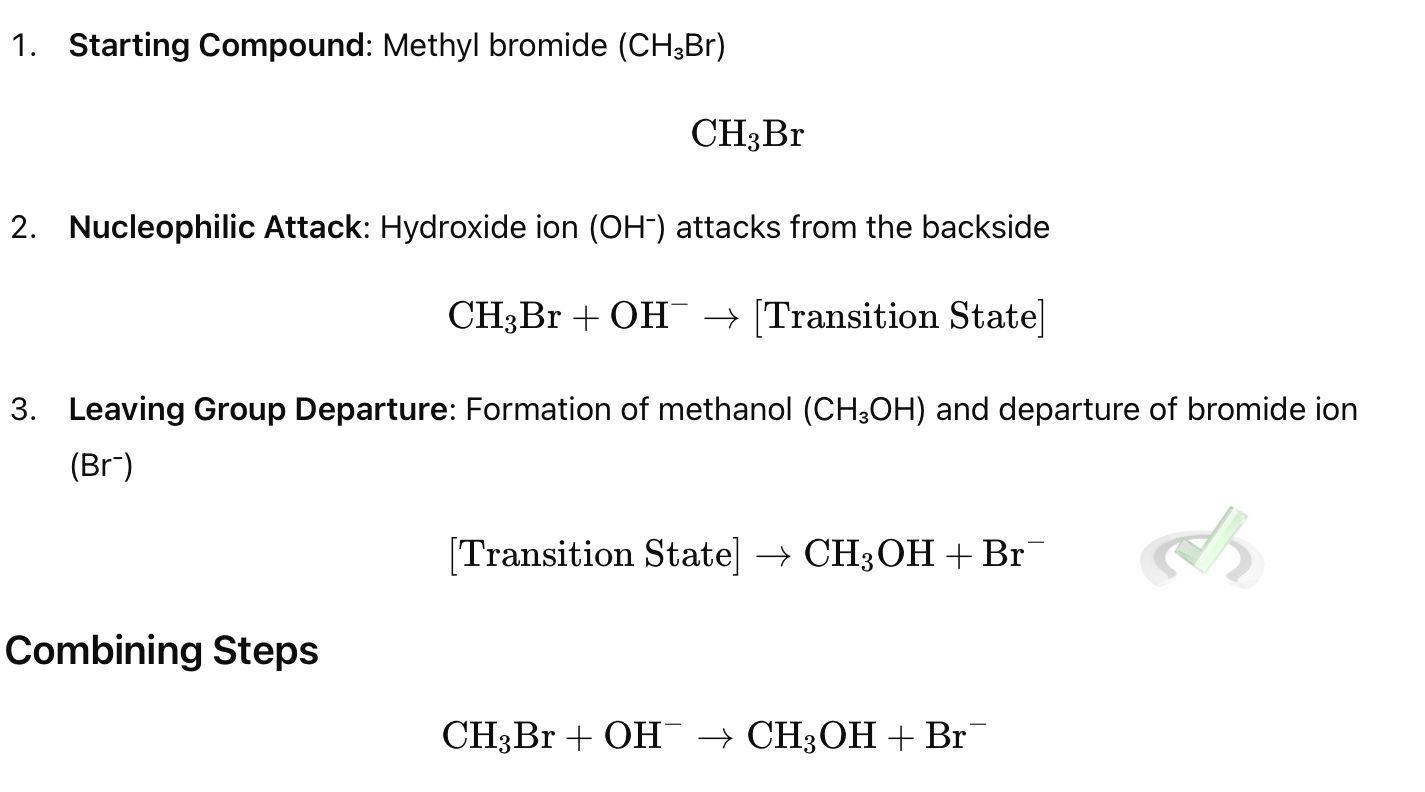
Rate
The rate of an SN2 reaction depends on both the nucleophile and the substrate. It follows second-order kinetics. This means the reaction rate is proportional to the concentrations of both the nucleophile and the substrate. This can be written as rate = k [nucleophile][substrate], where k is the rate constant.
Factors Affecting SN2:
- Substrate Structure: Primary substrates (carbon bonded to one other carbon) are more reactive than secondary substrates. Tertiary substrates (carbon bonded to three other carbons) are generally unreactive in SN2 reactions because of steric hindrance. Steric hindrance means bulky groups around the reactive carbon make it harder for the nucleophile to reach and react with it.
- Nucleophile Strength: Strong nucleophiles, like hydroxide ion (OH-) or cyanide ion (CN-), favor SN2 reactions. Strong nucleophiles are highly reactive and readily donate their electron pair to form a new bond.
- Solvent: Polar aprotic solvents, which do not form hydrogen bonds, are ideal for SN2 reactions. Examples include acetone, dimethyl sulfoxide (DMSO), and acetonitrile. These solvents do not solvate the nucleophile strongly, keeping it reactive.
SN1 Reaction
An SN1 (Substitution Nucleophilic Unimolecular) reaction is a two-step process in organic chemistry where a nucleophile replaces a leaving group. The rate of reaction depends on the concentration of the substrate.
Step 1: Formation of the Carbocation
The leaving group (LG) departs from the substrate (R-LG), forming a carbocation (R⁺). This is the slow, rate-determining step.
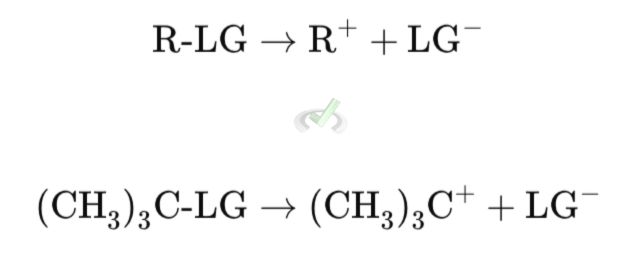
Step 2: Nucleophilic Attack
The nucleophile (Nu⁻) attacks the carbocation (R⁺), forming the final product.
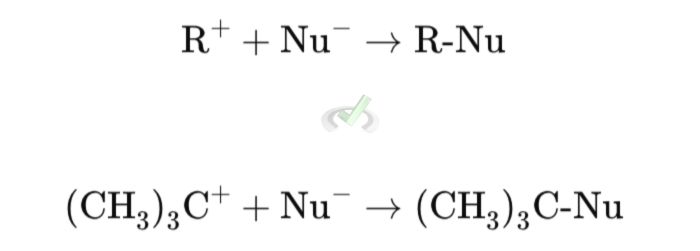
Example:
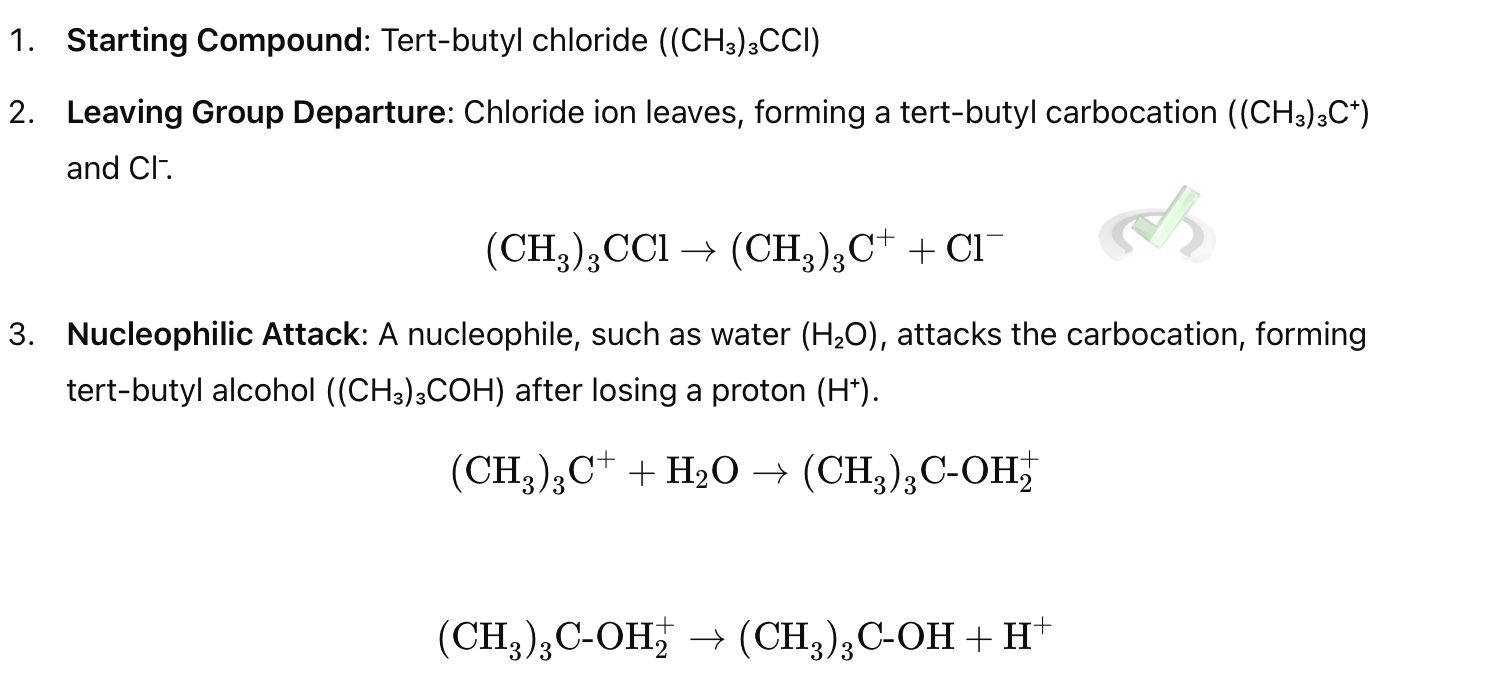
Rate
The rate of an SN1 reaction depends only on the concentration of the substrate. It follows first-order kinetics. This means the reaction rate is proportional to the concentration of the substrate. This can be written as rate = k [substrate], where k is the rate constant.
Factors Affecting SN1
Substrate Structure: Tertiary substrates form more stable carbocations and are more reactive in SN1 reactions. Primary substrates rarely undergo SN1 reactions because the resulting carbocations are highly unstable. Carbocation stability is influenced by the ability to spread the positive charge through inductive effects or resonance.
Leaving Group: Good leaving groups, such as iodide (I-) or tosylate (TsO-), facilitate SN1 reactions. A good leaving group can stabilize the negative charge after it leaves.
Solvent: Polar protic solvents, which can form hydrogen bonds, stabilize the carbocation intermediate, and are ideal for SN1 reactions. Examples include water, ethanol, and methanol. These solvents stabilize the transition state and intermediate by solvating the positively charged carbocation.
II. Comparison of SN2 and SN1 Reactions
III. Practical Applications and Examples
Understanding the differences between SN2 and SN1 reactions helps predict outcomes in organic synthesis. Here are some practical examples:
A. Laboratory Synthesis
SN2: Converting an alkyl halide to an alcohol using a strong nucleophile like sodium hydroxide (NaOH).
Reaction: R−Br+NaOH→R−OH+NaBr
The strong nucleophile, OH-, attacks the carbon atom from the opposite side of the bromine, displacing it in a single step.
SN1: Converting an alkyl halide to an ether using ethanol in a polar protic solvent.
Reaction: R−Br→EtOHR−OEt+HBr
Details: The reaction proceeds through the formation of a carbocation intermediate. Ethanol, acting as a nucleophile, attacks the carbocation, resulting in the formation of an ether.B. Biological Reactions
SN2 in Enzymes: Many enzymatic reactions involve SN2 mechanisms, where a nucleophile in the enzyme attacks the substrate directly. For example, the enzyme hexokinase catalyzes the phosphorylation of glucose by ATP through an SN2 mechanism.
Example: Glucose + ATP → Glucose-6-phosphate + ADP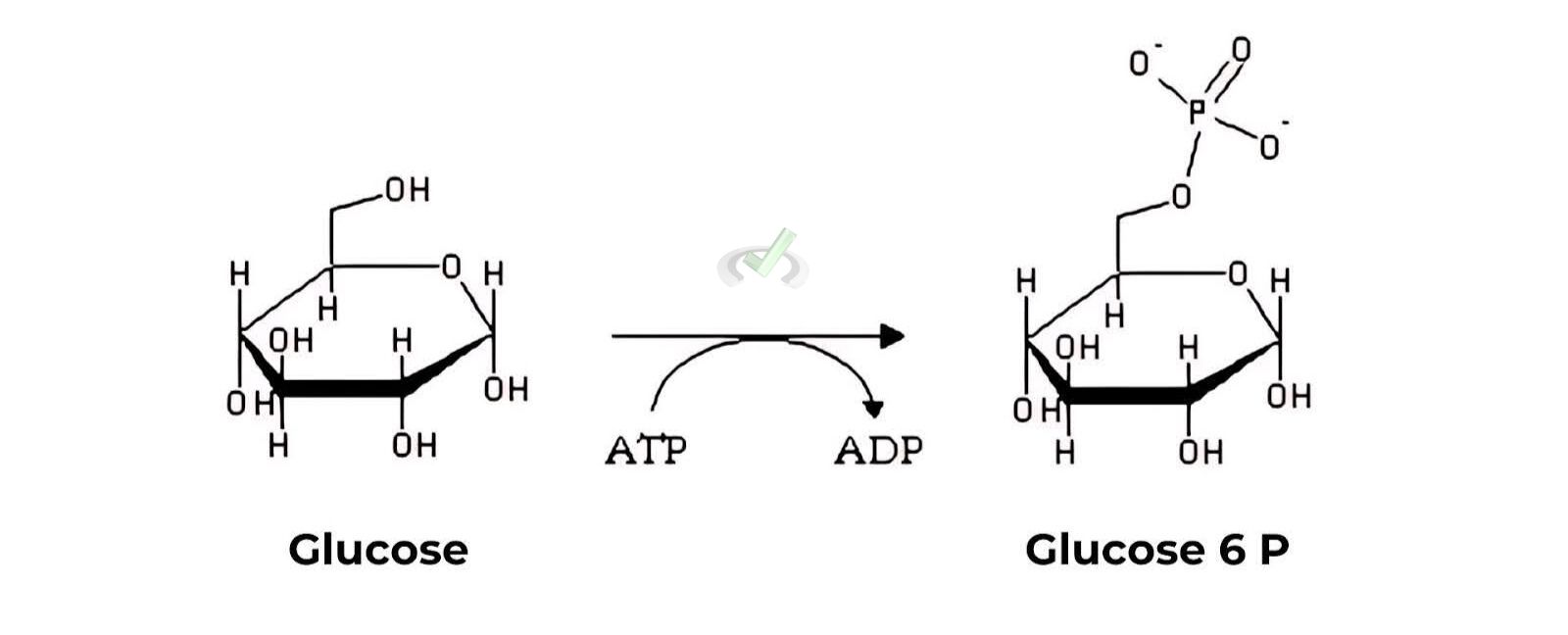
SN2 in Enzymes: Many enzymatic reactions involve SN2 mechanisms, where a nucleophile in the enzyme attacks the substrate directly. For example, the enzyme hexokinase catalyzes the phosphorylation of glucose by ATP through an SN2 mechanism.
Example: Glucose + ATP → Glucose-6-phosphate + ADP
IV. Connecting to Broader Organic Chemistry Concepts
A. Reaction Mechanisms and Synthesis
Knowing the mechanism helps in designing synthetic pathways. For instance, choosing between SN2 and SN1 can affect the stereochemistry of the product.
Example: In drug synthesis, the choice of reaction pathway can influence the drug's effectiveness due to stereochemistry.B. Predicting Reaction Outcomes
Understanding whether a reaction follows SN2 or SN1 helps predict the products, especially in complex molecules.
Example: In pharmaceutical chemistry, predicting the outcome of reactions ensures the correct formation of active ingredients. For instance, in synthesizing an active pharmaceutical ingredient (API). Knowing that a reaction follows an SN1 mechanism can help predict potential side products and optimize reaction conditions to increase yield.V. Wrap-Up and Key Terms
SN2 and SN1 reactions are key nucleophilic substitution mechanisms in organic chemistry. Understanding these mechanisms helps predict reaction outcomes and is crucial for successful synthesis in laboratory and biological settings.
Key Terms:
- SN2 Reaction: A one-step nucleophilic substitution with a backside attack and inversion of configuration.
- SN1 Reaction: A two-step nucleophilic substitution with a carbocation intermediate and potential for racemization.
- Nucleophile: A chemical species that donates an electron pair to form a bond.
- Leaving Group: A group that can leave the substrate, taking an electron pair with it.
- Polar Aprotic Solvent: Solvent that does not form hydrogen bonds, ideal for SN2.
- Polar Protic Solvent: Solvent that can form hydrogen bonds, suitable for SN1.
VI. Practice Questions
Sample Practice Question 1
What type of substrate is most reactive in SN2 reactions?
A) Tertiary
B) Secondary
C) Primary
D) Quaternary
Ans. C
Primary substrates have less steric hindrance, making them more reactive in SN2 reactions.
Sample Practice Question 1
Which solvent is ideal for SN1 reactions?
A) Acetone
B) DMSO
C) Water
D) Hexane
Ans. C
Water is a polar protic solvent, which stabilizes the carbocation intermediate in SN1 reactions.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot