Aldehydes and Ketones are functional groups with a carbonyl group attached to their carbon chain. Carbonyl groups comprise a carbon atom with a double bond with oxygen. They usually form through the oxidation of alcohol. Nucleophilic addition is a reaction wherein a nucleophile bonds with an electrophilic carbon atom. Aldehydes and Ketones undergo these reactions since they both have electrophilic carbonyl atoms.
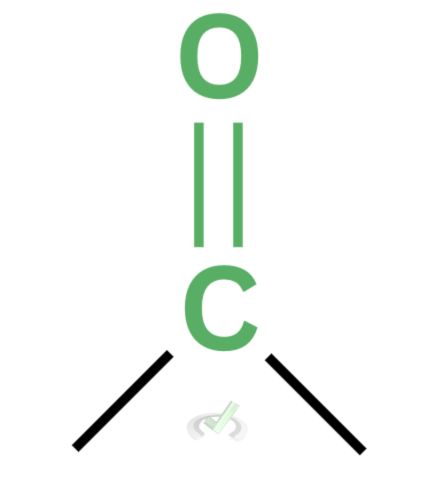
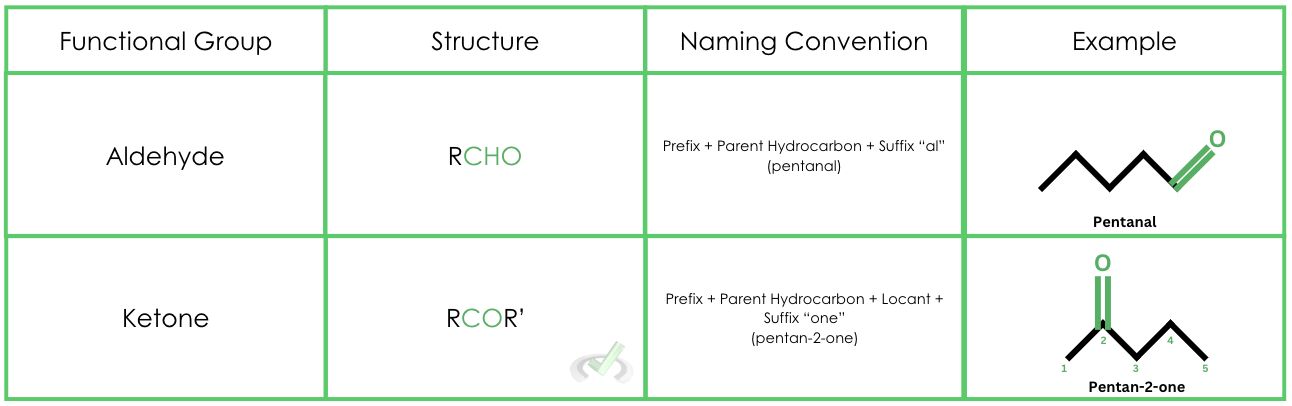
Let’s first discuss what aldehydes and ketones are. An aldehyde is a functional group with a carbonyl group at the end of a hydrocarbon chain. In contrast, ketones have a carbonyl group in the middle of a hydrocarbon chain. The typical structure of an aldehyde is taken as RCHO and ROR for a ketone, with R representing a hydrocarbon chain.
It’s important to note the main difference between nucleophilic addition reactions on aldehydes and ketones. Aldehydes are reduced into a primary alcohol, whereas ketones are reduced into a secondary alcohol. In primary alcohols, the -OH group is attached to a carbon atom bonded to one other carbon atom, and for secondary alcohols, the -OH group is attached to a carbon atom bonded to two other carbon atoms.
Here’s how we can identify an aldehyde from a ketone:
I. The Carbonyl Group
Various reactions occur in aldehydes and ketones because of the carbonyl group attached to them. Carbonyl groups have a unique characteristic: they’re electrophilic and partially positive. This means that they love electrons. In the double bond between carbon and oxygen, electrons tend to move towards oxygen because it has high electronegativity. Since the electron density tends to move towards oxygen, it leaves the carbon partially positive. This partial positivity makes the carbon atom want electrons (electrophilic).
Nucleophiles are electron-rich and are highly attracted to species that lack electrons. In short, nucleophiles seek out electrophiles. Carbonyl groups are electrophilic due to their partially positive charge. At the same time, nucleophiles are electron-rich. When an aldehyde or ketone is exposed to a nucleophile, the nucleophile attacks the electrophilic carbon atom. As this happens, the oxygen breaks a bond and forms another bond with a positive ion.
II. Mechanism
1. Nucleophilic Addition
The first stage of the process occurs by exposing propanal to sodium borohydride, which acts as the reducing agent. In this phase, our nucleophile is the hydrogen atom from sodium borohydride. Because it bears a negative charge and carbon is partially positive due to oxygen pulling more electrons towards itself, the negatively charged hydrogen atom from sodium borohydride is attracted to the partially positive carbon atom. The nucleophile attacks the electrophilic carbon.
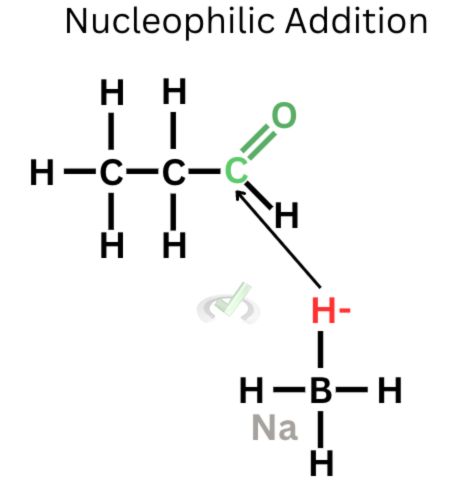
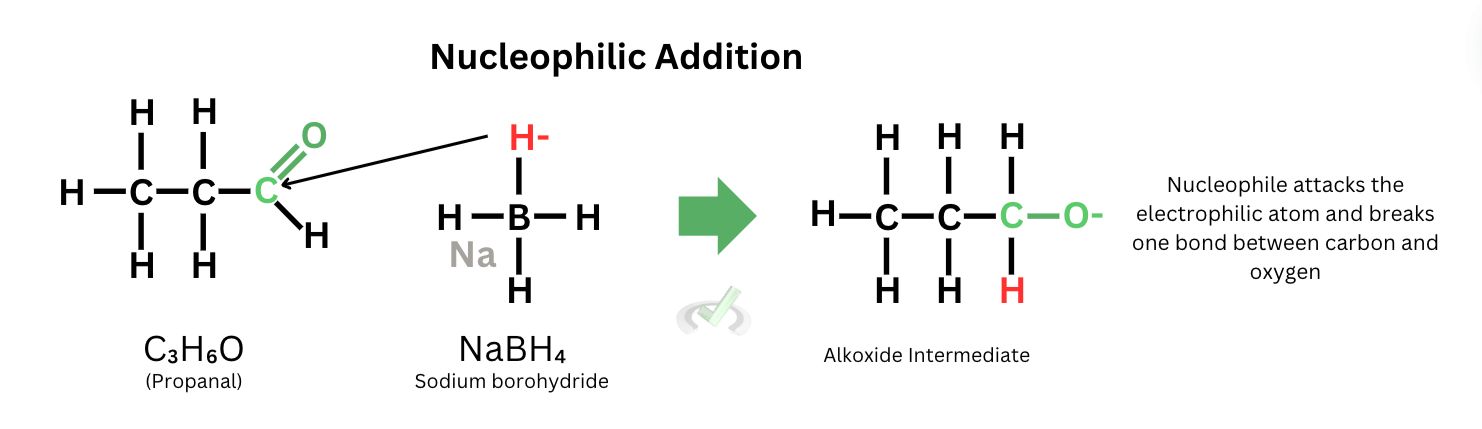
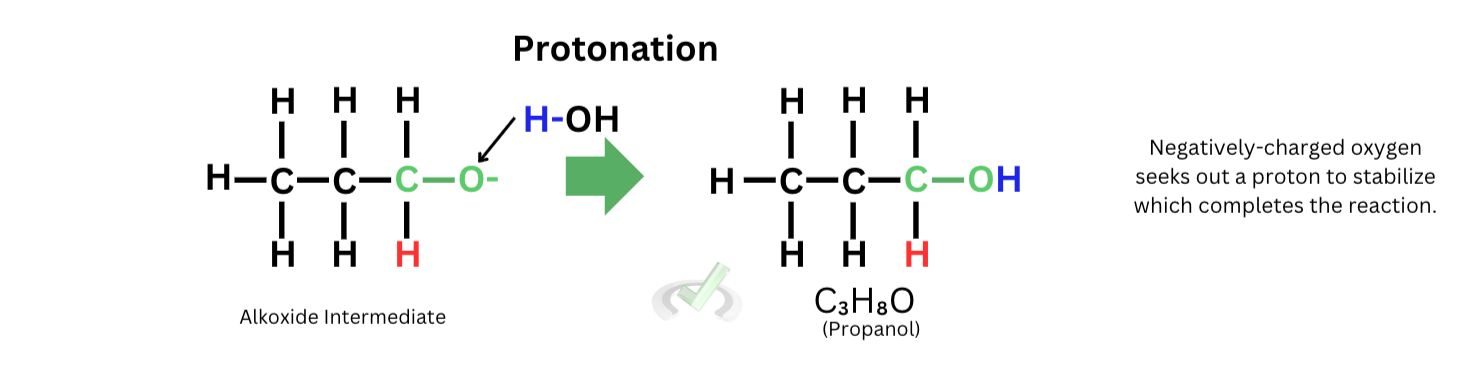
2. Protonation
As hydrogen attaches itself to the carbon atom, the double bond from oxygen breaks since carbon can only hold four bonds. This leaves the oxygen to bear a negative charge. To seek stability, the oxygen now needs a proton (H+). In the laboratory, once we add the reducing agent and the propanal, we usually put in an acid or water to quench the reaction. Thus, the hydrogen atom will be the proton source for the oxygen. The compound is stabilized once the hydrogen attaches itself to the oxygen atom, and the reaction is finally completed.
III. Common Nucleophilic Reactions
A. Hydration
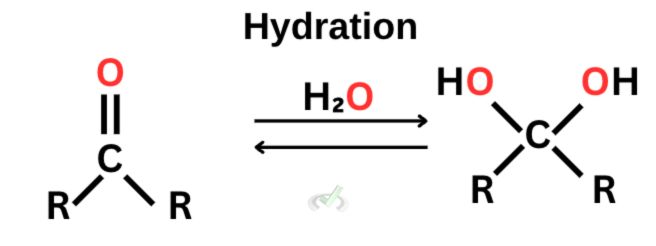
Hydration is a type of nucleophilic addition using water to react with aldehydes and ketones. In this process, a geminal diol or hydrate is formed. Geminal diols are alcohols that have carbon attached to two OH groups with a single bond. This can occur under neutral, basic, or acidic conditions.
Since carbon is electrophilic, it gets electrons from water. The nucleophile (oxygen) will attack the electrophilic atom. One bond breaks from the double bond as this happens. Hydrogen will continue to attach itself to the negatively charged oxygen to stabilize the whole molecule.
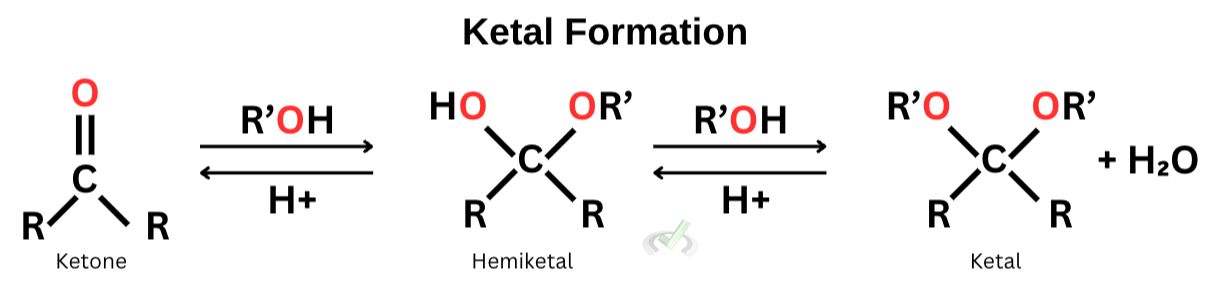
B. Acetal/Ketal Formation
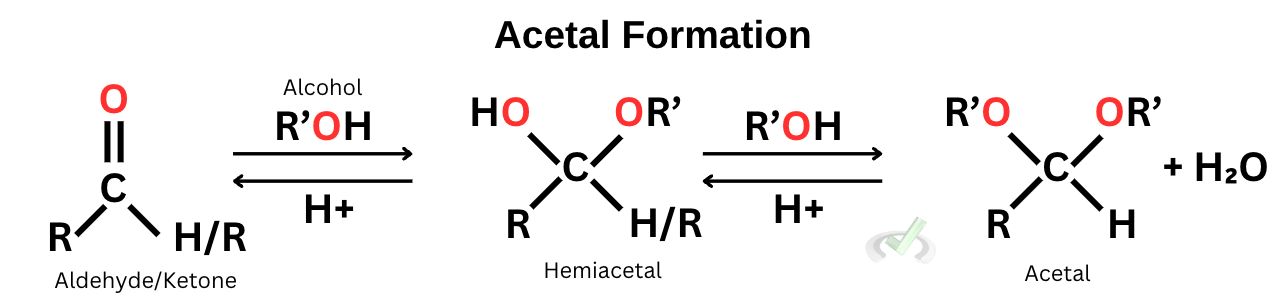
We previously discussed that a carbonyl group is highly electrophilic due to the partially positive carbon. Since the electronegativity of oxygen is higher, the electron density tends to move towards oxygen–leaving the carbon to have a partially positive charge. This makes the carbonyl atom extremely susceptible to attack from surrounding nucleophiles. To counter this, we have to protect the carbonyl atom from the nucleophiles, and one way to do this is by using alcohol (R’OH) to transform carbonyl groups into acetals.
Acetals are ideal, especially if we want to isolate our ketones and aldehydes when other functional groups are present in a solution. They are not prone to nucleophilic attack and react to oxidizing and reducing agents. As you will see, they can also be easily removed from a solution.
The mechanism of acetal formation starts with oxygen attacking the carbonyl carbon, forming a hemiacetal. With the presence of another alcohol group, the -OH group will be protonated, ultimately leading to an acetal. The mechanism is the same for all of the other nucleophilic addition reactions.
If we want to transform an acetal back into a hemiacetal, we can add water to the acetal so the reaction shifts to the left. If we want more acetal products, we can reduce water so the reaction shifts to the right. This phenomenon is explained by Le Chatelier’s principle, which suggests that a change in a variable in a system will cause a change in the direction of the equilibrium to counteract the initial change that we have made.
In the reaction below, we see two arrows pointing in opposite directions, a symbol of reversibility. When we add more water to our product, we encourage the reaction to shift towards the left side, producing more hemiacetals. When we reduce water, we initiate the production of more acetals.
C. Cyanohydrins
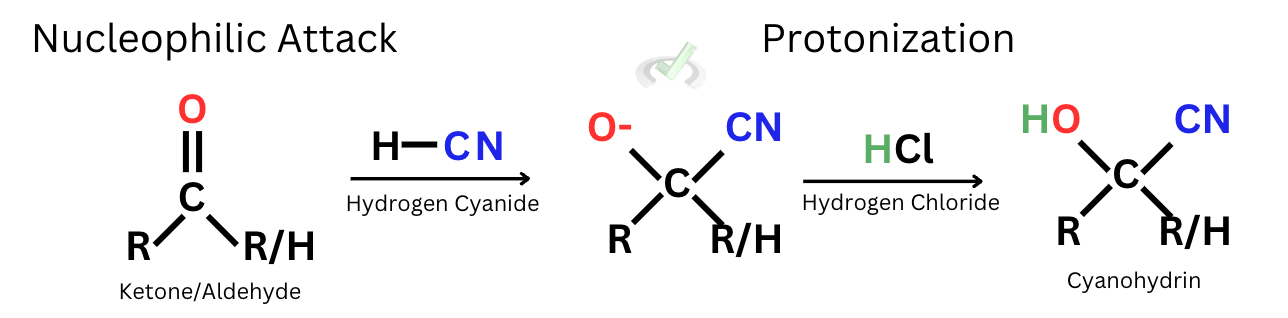
The formation of cyanohydrins happens when the nucleophilic attack is caused by cyanide. The mechanism starts with the nucleophilic attack on the carbonyl carbon, which leads to the double bond breaking and leaving oxygen with a negative charge. This will proceed to be protonated by a hydrogen atom, which can sometimes come from hydrogen chloride. Once the oxygen is protonated, the cyanohydrin is formed.
IV. Conclusion
Carbonyl groups are highly susceptible to nucleophilic attack due to the electronegativity of oxygen. During nucleophilic addition, a nucleophile attacks the carbon of an aldehyde or a ketone, which breaks the double bond between carbon and oxygen. After this, the oxygen atom bears a negative charge, and the nucleophile successfully bonds with carbonyl carbon. Since oxygen will always seek to stabilize itself, protonation occurs, which yields the final desired product. Hydration, acetal formation, and cyanohydrin formation are common types of nucleophilic addition. Hydration uses water as the nucleophile, acetal formation uses alcohol, and cyanohydrin formation uses hydrogen cyanide as a nucleophile. These processes are essential in the laboratory since we can get our desired product using nucleophilic addition.
V. Key Terms
- Acetal/Ketal - An acetal is a functional group with two oxygen atoms bonded to a carbon atom. Acetals used to be a singular term for species derived from aldehydes and ketals from ketones; however, chemists now use acetal to describe both.
- Alkoxide intermediate - A functional group that forms as an intermediate species in various reactions.
- Cyanohydrin - a functional group wherein a carbon is bonded to a cyanide and hydroxyl group.
- Electrophile - A species that is electron-deficient.
- Le Chatelier’s Principle: “If change is applied to a system at equilibrium, the equilibrium position will shift in the direction which reduces the stress.”
- Nucleophile - A species that is electron-rich and donates electrons to electron-deficient species.
VI. Practice Questions
Sample Practice Question 1
If we react Acetone (C₃H₆O) with lithium aluminum hydride (LiAlH₄) and quench it with water, which of the following products will we most likely get?
A. 1-Propanol
B. 2-Propanol
C. 3-Propanol
D. Propane
Ans. B
Sample Practice Question 2
Aldehydes and Ketones are reduced into primary and secondary alcohols, respectively. Which of the following statements likely follows?
A. Aldehydes and ketones undergo oxidation to form alcohols because they gain electrons during nucleophilic addition.
B. Aldehydes and ketones do not undergo oxidation to form alcohols because they gain protons from a basic solution.
C. Aldehydes and ketones undergo reduction because they gain protons from an acidic solution.
D. Aldehydes and ketones result from oxidation of primary and secondary alcohols, respectively.
Ans. D








 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot