Covalent bonds form by sharing electrons. This happens when orbitals overlap to form a bond. These bonds occur between nonmetals.
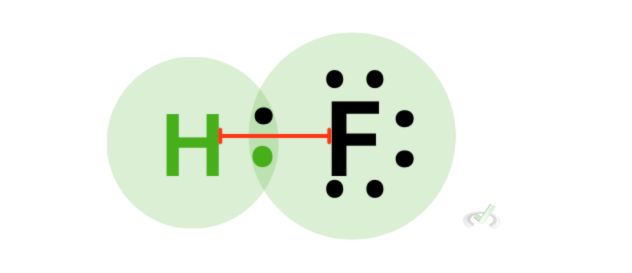
When we look deeper into covalent bonding, we see that the sharing of electrons happens due to the overlap between orbitals. Let’s take hydrogen fluoride as an example.
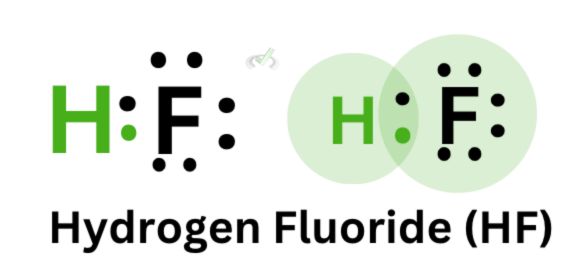
Hydrogen and fluorine are nonmetals. Hydrogen belongs to group 1, which means it has 1 valence electron, and fluorine is in group 17, which means it has 7 valence electrons. Remember that we count valence electrons by counting the groups and skipping the transition metals starting from group 1.
When hydrogen and fluorine form a bond, their orbitals overlap, causing stability between the two atoms. Since hydrogen follows the duet rule, it is already stable with two electrons; unlike ionic bonding, where the electron from metal transfers to a nonmetal, covalent bonding involves sharing electrons through the overlap of their orbitals.
We see this in the overlap between the orbitals of hydrogen and fluorine to form a bond.
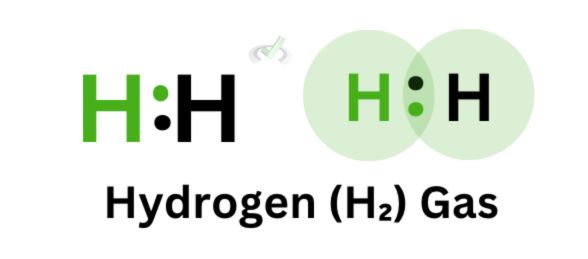
Another example we can look at is hydrogen gas. A bond forms between two hydrogen atoms since hydrogen is stable with two electrons. The overlap of their orbitals results in the formation of a covalent bond.
Polarity of Covalent bonds
One distinction you might notice between hydrogen fluoride and hydrogen gas is that in hydrogen fluoride, fluorine seems to be electron-heavy, whereas, for hydrogen gas, the electron distribution seems equal.
This concept in bonding might be helpful, and it is called polarity.
When an atom is electronegative, it tends to attract electrons towards itself. In hydrogen fluoride, fluorine is more electronegative (fun fact: it’s the most electronegative atom!) than hydrogen; this is why the valence electron of hydrogen is drawn to fluorine and not the other way around.
Since the electron distribution is unequal for hydrogen fluoride, we can say that hydrogen fluoride is a polar molecule. One side has a lot of electrons (fluorine), and the other lacks electrons. We have a partially negative side on fluorine and a partially positive side on hydrogen.
On the other hand, the electron distribution for hydrogen gas is even. No element receives more electrons than the other. Hydrogen gas is a nonpolar molecule since both atoms have the same electronegativity.
Polarity matters in a lot of cases. We see polar molecules affecting molecular behavior by influencing intermolecular forces as a result of this uneven distribution of molecules.
I. Sigma bonds and Pi bonds
Covalent bonding differs from ionic bonding because it involves the overlap of orbitals to share an electron. Let’s take ethane (C2H6) as an example.
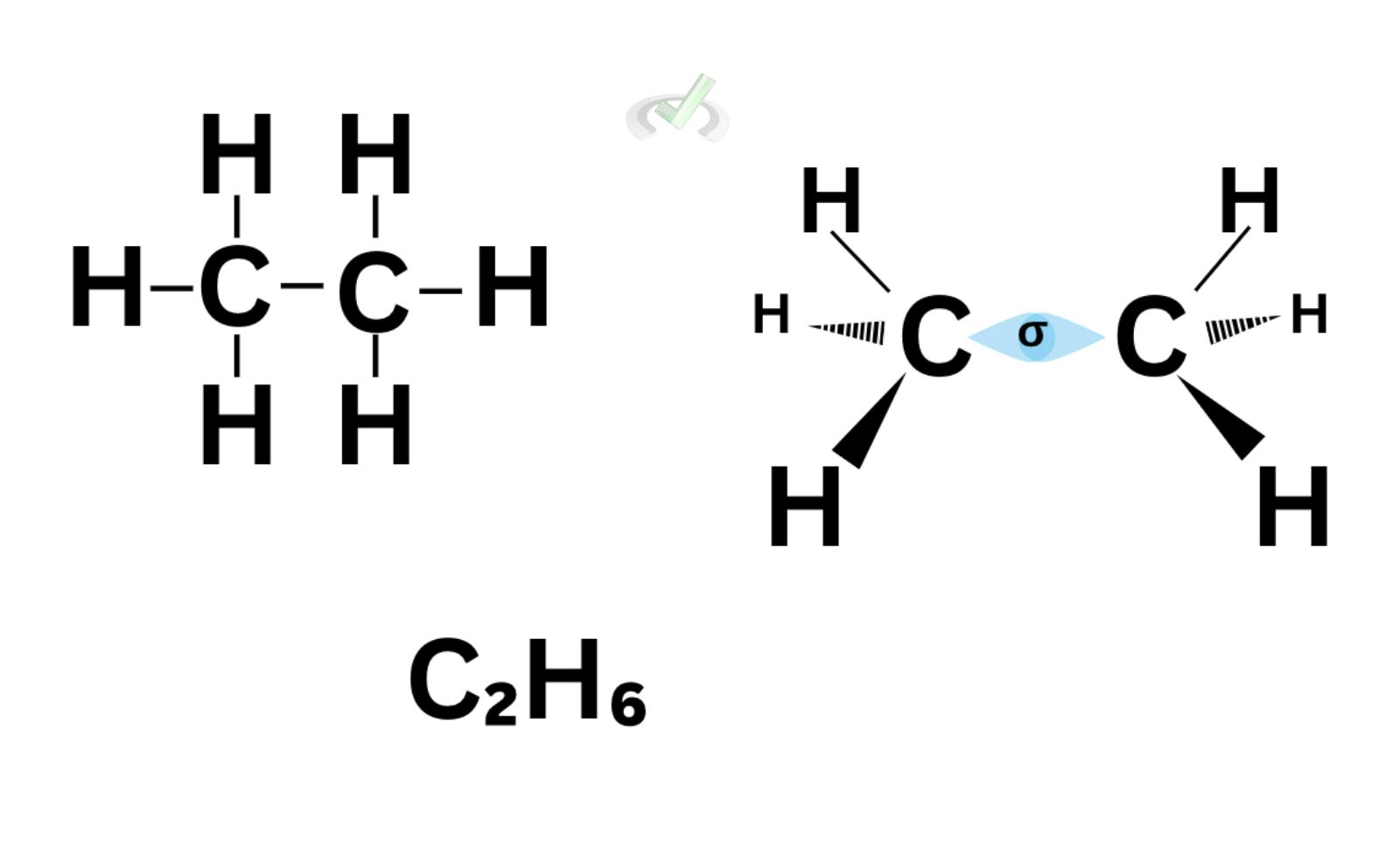
Here, a carbon atom is bonded to another carbon using a single bond. A single bond happens when the orbitals of the carbon atoms overlap. When this happens, a sigma bond forms between the two carbon atoms. This indicates that the electrons could be found in the overlap of the orbitals of the carbon atoms.
A sigma bond forms when the orbitals overlap to share an electron. This can be seen in any single bond. For ethane, we see the sigma bond between the two carbon atoms through the overlap of their orbitals.
Now, how about double and triple bonds?
To explain multiple bonds, let’s take ethene (C2H4), a double-bonded hydrocarbon, as an example.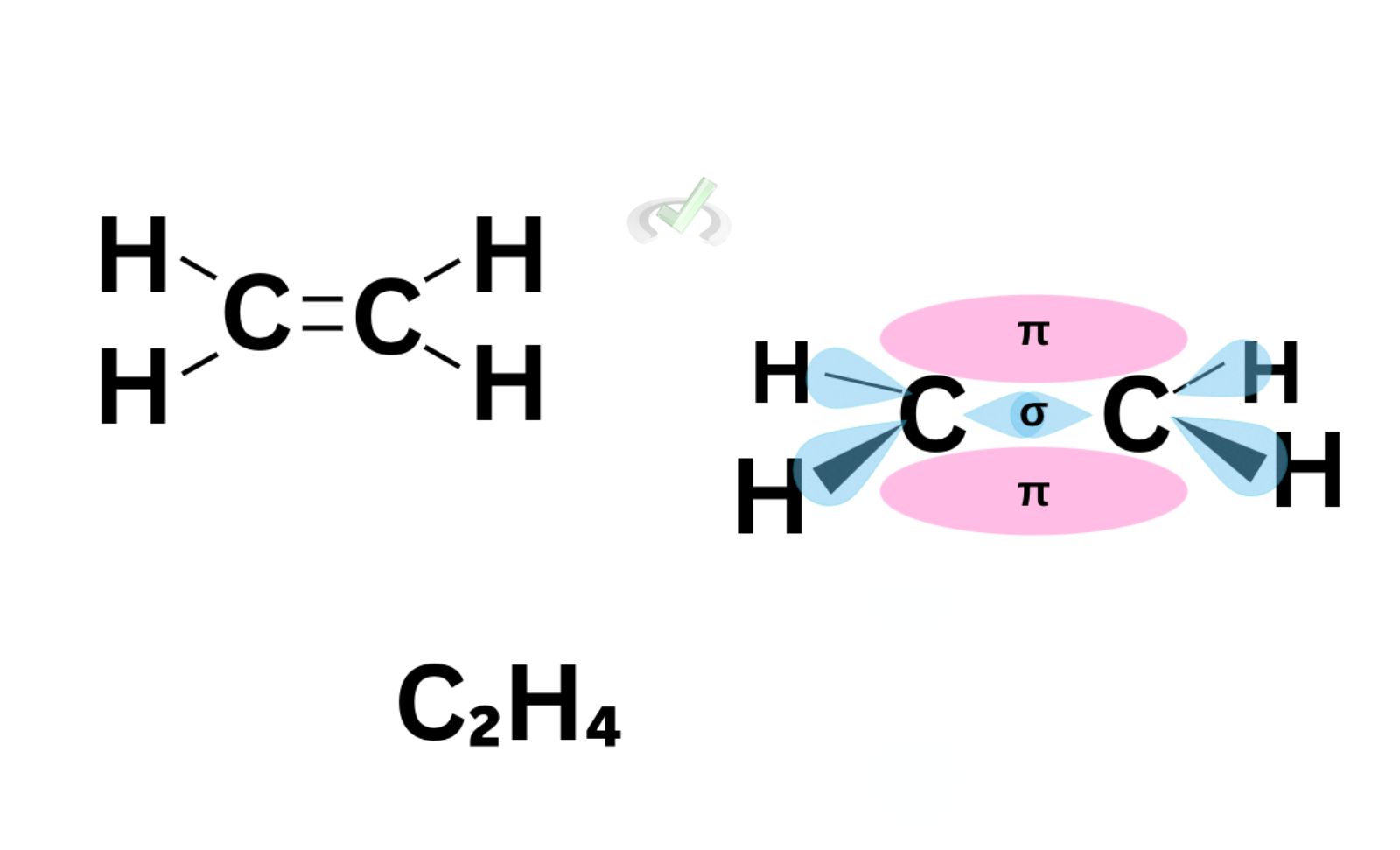
If we solely look at ethene’s Kekule structure, we’ll immediately notice a double bond between the two carbon atoms. Since one bond means there are two electrons involved, we have fewer hydrogen atoms this time to still follow the octet rule and leave each carbon atom with 8 valence electrons.
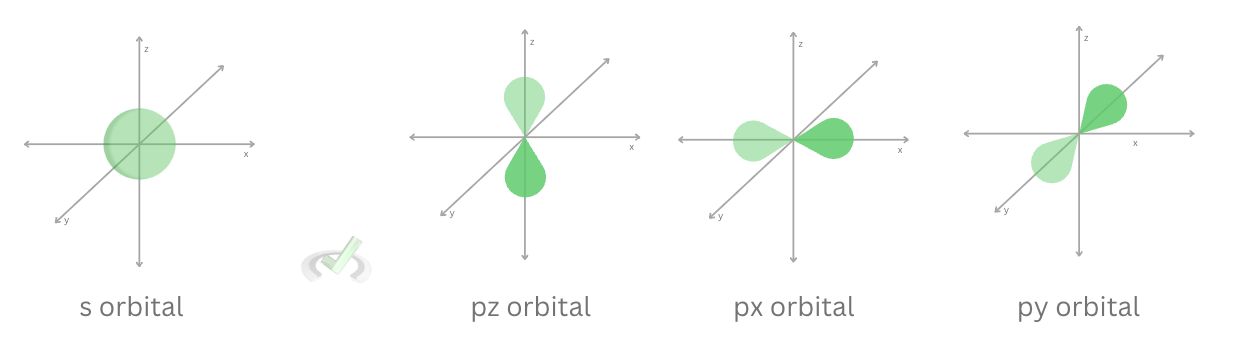
Now, sigma bonds are known as a linear overlap. This is the sideways overlap of an atom’s orbital with another orbital. From our previous discussions, we’ve established that the s orbital is spherical, so it has only one orientation. The p orbital, on the other hand, has three possible orientations. It has a dumbbell shape and can be found along the x, y, and z-axis. When electrons are attracted sideways, a pi bond forms.
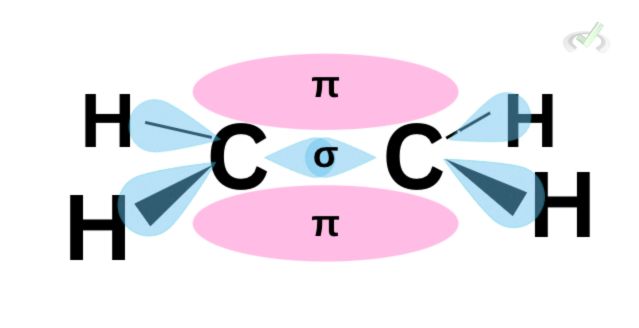
For ethene, a sideways overlap of these p orbitals happens; the regions we see in pink are the clouds or areas shared between the second bonding electron pair of a bonding atom. While we cannot locate which area in these lobes the electrons are, we can say that the pi region is where another electron pair forms a bond. The area of this overlap is where the pi bond forms.
The same rule applies to the formation of a triple bond. A triple bond would have one sigma bond and two pi bonds as a result of the sideways overlap of the orbitals. The area above and below the sigma bond is where the two electron pairs may be.
No. of sigma bonds | No. of sigma bonds | |
|---|---|---|
Single bond 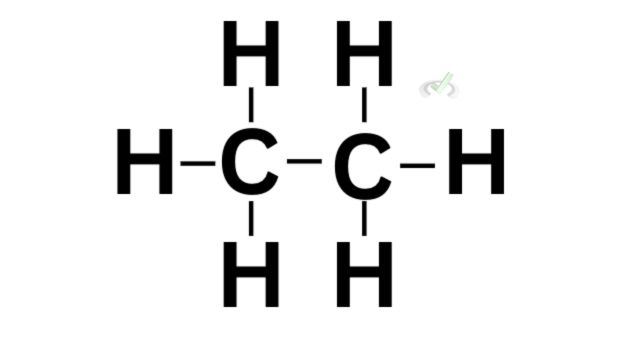 | 1 | 0 |
Double bond 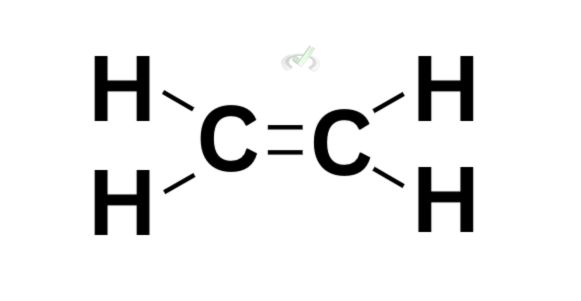 | 1 | 1 |
Triple bond 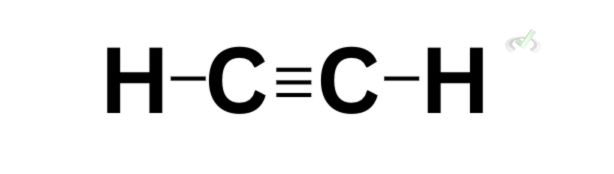 | 1 | 2 |
II. Bond Length and Bond Strength
Single bonds
Single bonds are the longest since they share only one pair of electrons between two atoms. The overlap of the orbital takes less space since it only allows the pairing of two electrons. While single bonds are the longest, they are also the easiest to break, which means they are the weakest. It has the lowest dissociation energy, which means that it only takes a few energy to break a single bond.
Double bonds
Triple bonds are the shortest among the three, Since three pairs of electrons (total of 6) are involved in the overlap of the orbitals, the pull between bonding atoms is the greatest. Similar to double bonds, triple bonds are much more difficult to break than double and single bonds. This is due to its dissociation energy being higher than the rest. This means that triple bonds are the strongest and have the shortest bond length.
Triple bonds
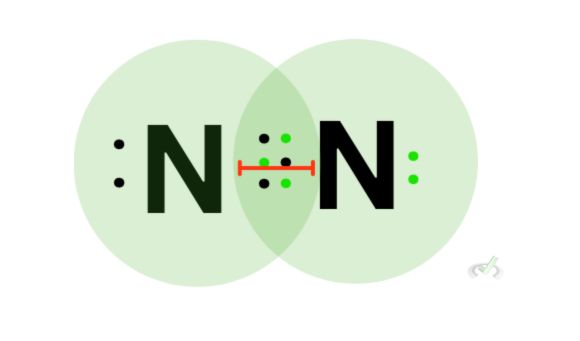
Triple bonds are the shortest among the three, Since three pairs of electrons (total of 6) are involved in the overlap of the orbitals, the pull between bonding atoms is the greatest. Similar to double bonds, triple bonds are much more difficult to break than double and single bonds. This is due to its dissociation energy being higher than the rest. This means that triple bonds are the strongest and have the shortest bond length.
III. Conclusion
A covalent bond forms through the sharing of electrons between nonmetals. This is possible since the bonding nonmetals’ orbitals overlap to form a bond. The linear overlap of two orbitals makes a sigma bond. The side-by-side overlap of a p orbital makes a pi bond. This is important because a single bond will only have one sigma bond. A double bond forms due to one sigma bond and one pi bond. A triple bond forms through one sigma bond and two pi bonds.
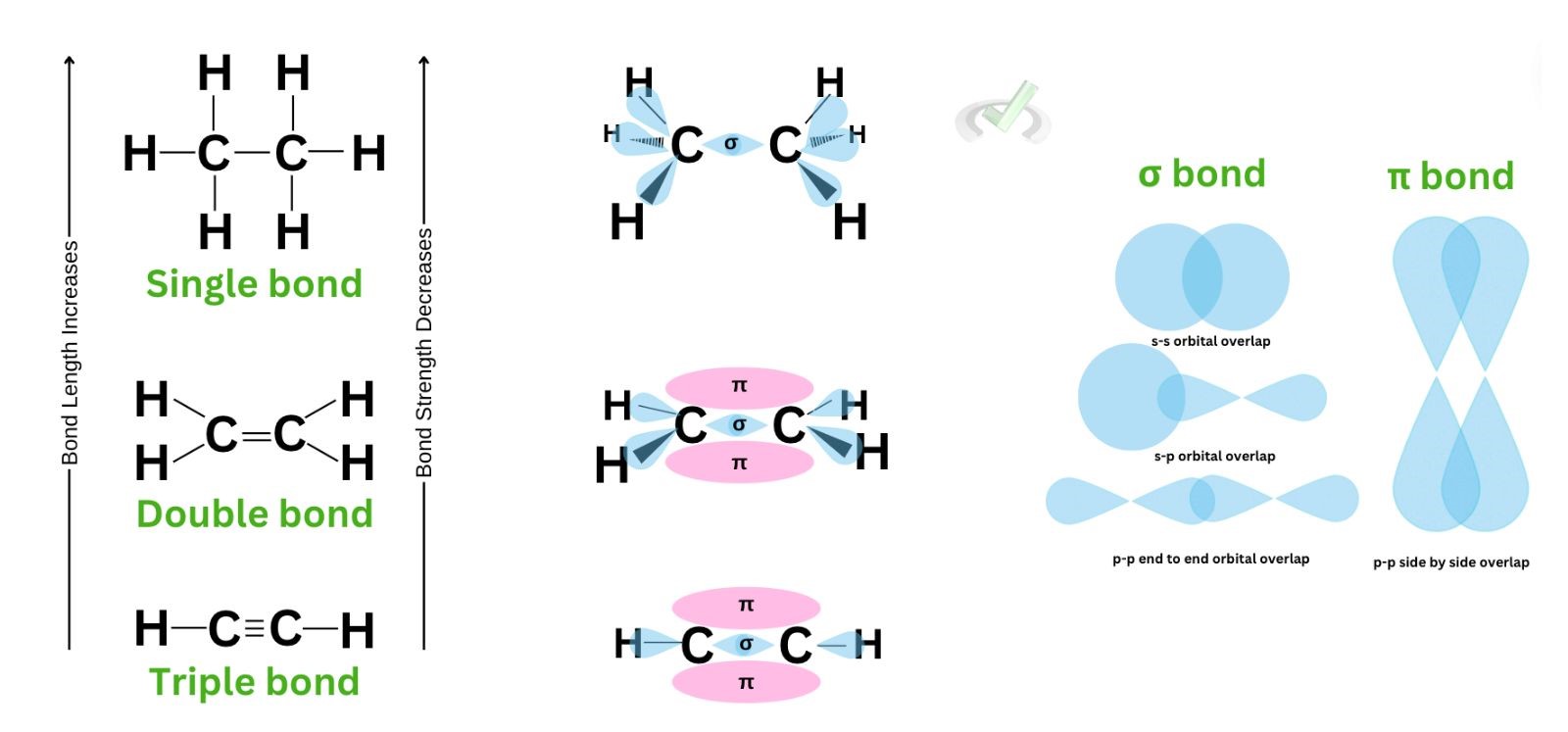
Single, double, and triple bonds differ in strength and length. Bond strength refers to the energy needed to break a bond, and bond length is the distance between the nucleus of two bonding atoms. A single bond has the weakest bond but is the longest. The triple bond is the strongest and has the shortest bond length. Double bonds have intermediate strength and bond length among the three. These properties–bond length and strength are influenced by the electron density in the overlap of the bonding atoms. The more electrons are present, the stronger the pull of the atoms towards each other, making the bond length much shorter.
IV. Key Terms
- Bond length - the distance between the nuclei of two bonding atoms.
- Bond strength - the energy needed to break a bond.
- Electron density - the distribution of electrons in an orbital.
- Nonpolar molecules - bonded molecules with equal electron density.
- Pi bond - a bond that forms when two p orbitals overlap side by side
- Polar molecules - molecules with unequal electron distribution.
- Sigma bond - a bond that forms when adjacent orbitals form an end-to-end overlap.
V. Practice Questions
Sample Practice Question 1
Arrange single, double, and triple bonds from weakest to strongest.
A. Single bond, double bond, triple bond
B. Triple bond, double bond, single bond
C. Single bond, triple bond, double bond
D. Triple bond, single bond, double bond
Ans. A
Sample Practice Question 2
Which of the following is incorrect?
A. All single bonds are sigma bonds
B. All sigma bonds are single bonds
C. All bonds have sigma bonds
D. Triple bonds have a sigma bond.
Ans. B







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 





















 reviews on TrustPilot
reviews on TrustPilot