I. What is the Electron Transport Chain?
We’re almost to the point where we can start making ATP! You’ve made it all the way from glycolysis, the PDH complex, and the citric acid cycle and now only have a couple more hurdles before you become one step closer to mastering carbohydrate metabolism!
While this is also a very high yield topic and one that the AAMC likes to play favorites with, the process only has 4 enzymes to memorize! Actually, we find that for the electron transport chain, it’s more about getting a wholescale view and trying to drown out the miniscule detail.
If you’ve taken a metabolic biochem class, we’ve got some good news! The probably in depth amount of information that you learned about the electron transport chain in your class will all be cut down, as we’ll try to include just all the necessary details you’ll need to know.
II. Electron Transfer in the Mitochondria
Y’all know the drill! Let's get a quick general breakdown of the ETC before we get into all the enzymes and associated processes!
A. Net Molecular and Energetic Results of Respiration Process
The electron transport chain (ETC) is a 4 enzyme complex embedded in the inner mitochondrial membrane which transfers the electrons obtained from NADH and FADH2 oxidation to power the pumping of protons across the membrane to generate a H+ electrochemical gradient.
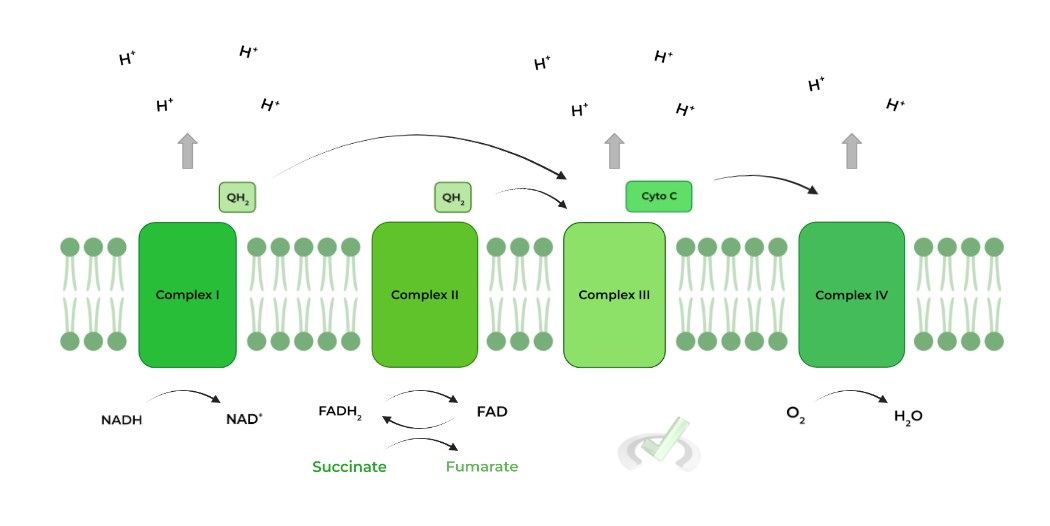
Hopefully now the term oxidative phosphorylation makes sense! As seen in the last complex, oxygen will be the final electron acceptor in this transfer of electrons, reducing it to water. We’ll cover this more in detail later in the article!
B. Electrochemical Gradient
We’ve tossed around this term but haven’t really clearly defined it until now! When the protons are pumped across into the intermembrane space, the electrochemical gradient is generated.
The “electro” component refers to the accumulated positive charge in the intermembrane from the protons; the “chemical” component just refers to the H+ ions.
During ATP synthesis, the protons will move DOWN their electrochemical gradient into the matrix so as to equally balance the charge (“electro”) and the H+ ion concentration (“chemical”).C. ETC Complexes
The ETC complexes are basically responsible for the transfer of electrons to oxygen, which is the final electron acceptor! Let’s take a look at the complexes one by one and their function!
I. Complex I: NADH Dehydrogenase
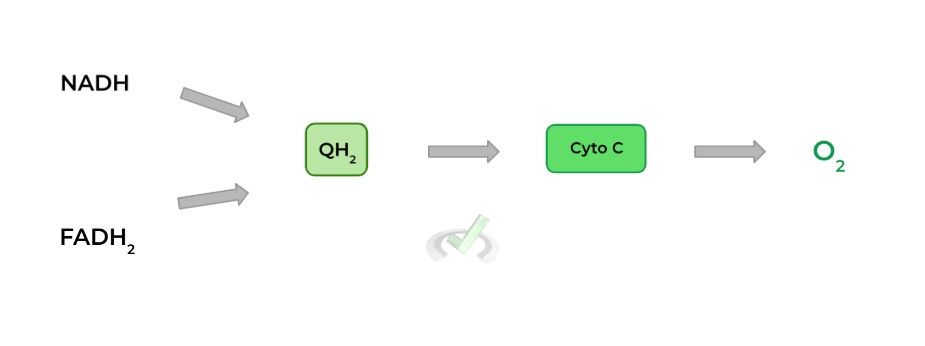
As suggested by the name, this complex oxidizes NADH back into NAD+. The electrons from the oxidized NADH are then transferred to a molecule called ubiquinone/coenzyme Q (Q) reducing it to ubiquinol (QH2).
Ubiquinol basically acts as an electron carrier which can move within the inner membrane transferring the electrons to complex III as we’ll cover soon.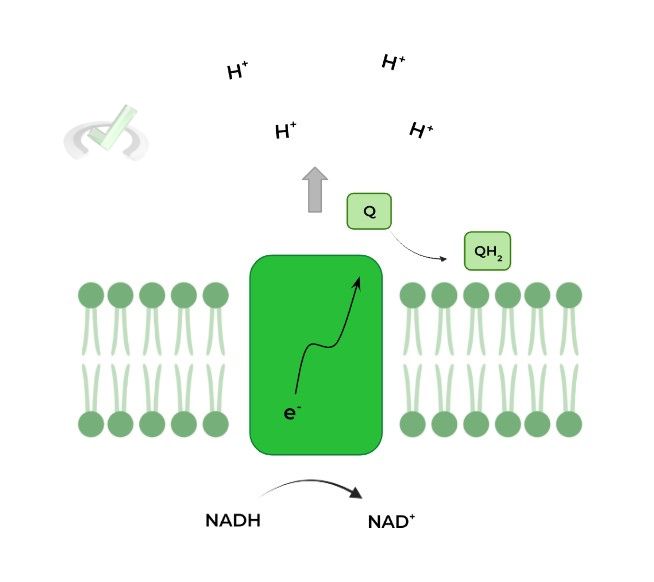
Additionally, complex I also results in the pumping of 4 protons across the membrane into the intermembrane space as a means to generate the H+ electrochemical gradient.
II. Complex II: Succinate Dehydrogenase
Getting deja vu? That’s cause we already saw this enzyme in the citric acid cycle! Recall that the enzyme oxidizes succinate to fumarate and reduces FAD to a FADH2.
However, the enzyme oxidizes FADH2 back to FAD to transfer the electrons to a molecule of ubiquinone reducing to ubiquinol, similar to complex I! The ubiquinol will then also transport those electrons to complex III.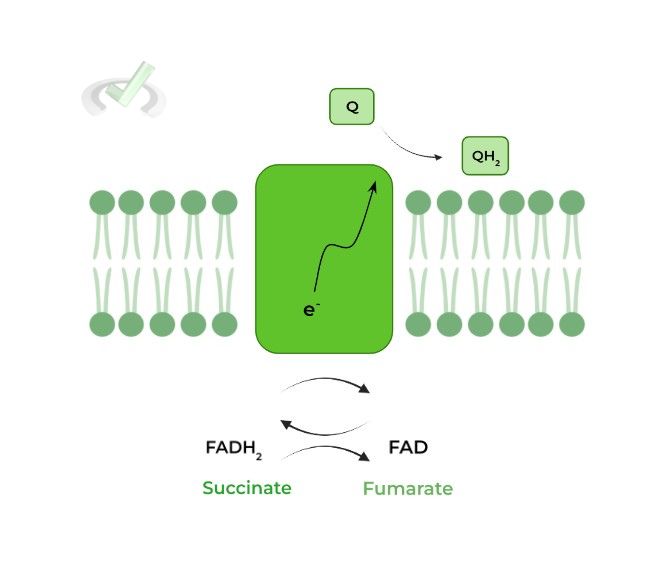
A unique feature about the complex is that it is the only complex that DOES NOT pump protons across the membrane. The complex rather contributes to the electrochemical gradient INDIRECTLY by generating QH2.
III. Complex III: Ubiquinol-Cytochrome C Oxidoreductase
Now we can see where ubiquinol comes into play! The QH2 produced from complex I & II travel within the membrane reaching complex III.
Again, another transfer of electrons occurs! QH2 essentially transfers its electrons to another electron carrier called cytochrome C, which functions similarly to QH2 as it carries the electrons to complex IV.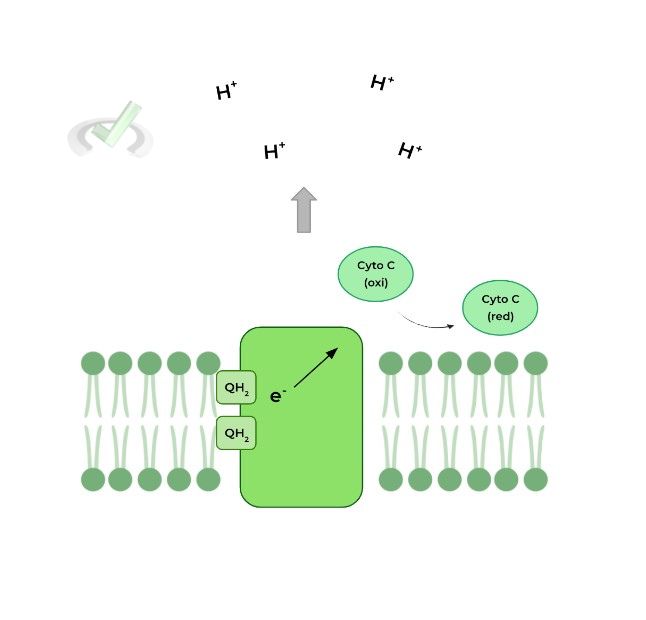
Similar to complex I, complex III also pumps 4 protons across into the intermembrane space to help generate the gradient.
IV. Complex IV: Cytochrome C Oxidase
We’re here at the final step! Here, the reduced cytochrome C molecules become oxidized and transfer their electrons to complex IV.
From here, the electrons are then transferred to the final electron acceptor, oxygen, which reacts with a couple of H+ to produce H20. Now it’s much clearer why the process is called oxidative phosphorylation.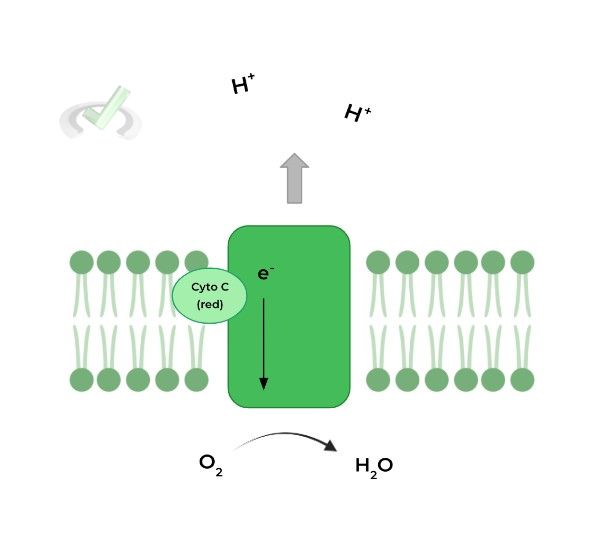
Similar to complex I and III, complex IV also pumps protons but in this case only pumps 2 H+ ions as opposed to the 4 that the other complexes pump.
Though your biochemistry class may have covered more, we’ve tried to boil it down to the most high yield & important information! Take a look at the diagram below for a simplified view of the flow of electrons in the ETC!
III. Bridge/Overlap
Why does the transfer of electrons happen in this specific order from ubiquinone to cytochrome C to oxygen? It all has to do with reduction potential – let’s quickly review the concept!
I. Standard Reduction Potential
The standard reduction potential is basically a value given to a molecule signifying how likely it is to accept electrons and thus be reduced. The “standard” term simply refers to this value under standard conditions (I.e. 1 atm, 298 K, etc.)
The more positive the value is, the MORE LIKELY the molecule will be reduced; the less positive the value, the LESS LIKELY it’ll be reduced.Molecule | E’˚ (V) |
|---|---|
Q | 0.045 |
Cytochrome C | 0.254 |
O2 | 0.816 |
Now it should also make sense why oxygen is the last electron acceptor as it’s reduced to water. Oxygen has the highest standard reduction potential (E’˚), which is why it’s the last electron acceptor.
You can also kinda see a flow: similar to how electrons pass from ubiquinol to cytochrome C to oxygen, the standard reduction potentials also increase in the same fashion.
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Net Molecular and Energetic Results of Respiration Process
The electron transport chain is a 4 enzyme complex which transports the electrons from the oxidized NADH and FADH2 molecules to oxygen as the final electron acceptor.
The process also allows for the pumping of protons across the membrane into the intermembrane space to generate the H+ electrochemical gradient.B. Electrochemical Gradient
Generated by the pumping of protons across the membrane into the intermembrane space. The “electro” refers to the accumulated positive charge and the “chemical” component refers to the high concentration of H+ within the intermembrane space.
The downhill flow of the H+ ions down their electrochemical gradient powers the synthesis of ATP via ATP synthase!C. ETC Complexes
The 4 complexes of the ETC transfers the electrons from the oxidized NADH and FADH2 to the final electron acceptor, oxygen. In addition, the 3 of the complexes pump protons across the membrane to generate the electrochemical gradient.
I. Complex I: NADH Dehydrogenase
Catalyzes the oxidation of NADH to NAD+. The released electrons are then transferred to ubiquinone (Q) to ubiquinol (QH2).
Ubiquinol then travels within the membrane and will then transfer the electrons to complex III. This complex allows for the pumping of 4 H+ ions across the membrane.II. Complex II: Succinate Dehydrogenase
This is the same enzyme involved in the citric acid cycle, catalyzing the oxidation of succinate to fumarate while reducing FAD to FADH2.
FADH2 is then reoxidized to FAD; the electrons gained from the oxidation are also transferred to a Q molecule, reducing to QH2, where it will also travel to transfer the electrons to complex III.
This is the only complex of the ETC that doesn’t pump protons across the membrane. In this case, it contributes INDIRECTLY by producing more QH2 molecules.III. Complex III: Ubiquinol-Cytochrome C Oxidoreductase
The QH2 generated from complex I and II arrive at complex III and transfer their electrons and reduce cytochrome C.
The reduced cytochrome C molecules function similarly to QH2, as it’ll move within the membrane to transport the electrons to complex IV.
Similar to complex I, this complex also pumps 4 protons across the membrane to aid in generating the gradient.IV. Complex IV: Cytochrome C Oxidase
This is the final complex of the ETC which takes the electrons coming from the cytochrome C and transfers the electrons to oxygen, the final electron acceptor.
With a couple of H+ and the electron transfer, oxygen is reduced to water. Like complex I and III, complex IV also pumps protons, but only pumps 2 H+ instead of 4.
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1:
During the electron transport chain, which of the following best describes the charge of the mitochondrial matrix?
A. Negative
B. Positive
C. Neutral
D. Cannot be determined
Ans. A
During the electron transport chain, the complexes, in addition to transporting the electrons, also allow for the pumping of protons across the membrane into the intermembrane space.
As such, the intermembrane space accumulates a positive charge while the mitochondrial matrix becomes more negative due to the exiting of H+ ions.
Sample Practice Question 2:
Which of the following would occur upon the inhibition of complex II of the electron transport chain?
I. Inhibition of the citric acid cycle
II. Decreased QH2 production
III. Accumulation of succinate
A. I only
B. III only
C. I and II
D. I, II, and III
Ans. D
Recall that in addition to being a part of the ETC and producing QH2, complex II (succinate dehydrogenase) is also a member of the citric acid cycle! As such, inhibition of this enzyme would cause a halting of the citric acid cycle.
In addition, because succinate dehydrogenase is inhibited, there will also be build up of succinate as succinate cannot be oxidized to fumarate.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
