I. What are the Structural Descriptions and Functions of Fatty Acids?
If you’re on a diet, you may be paying careful attention to them and how much of these fats you’re consuming! Additionally, you may even undergo tests to measure and monitor their levels in the blood. However, these molecules serve a crucial role in cellular function!
As we said, fatty acids have crucial roles in both cellular structural integrity and metabolism! Like all other things, it’s just important to make sure we have a good balance of not too much but also not too little in order to maintain physiological homeostasis.
When going through this article, you’ll again really get a good chance and grasp to see other content overlaps from different subjects all come together! As we always encourage here, make these connections and bridges as they’ll help you not only in studying but also come test day!
II. Importance of Fatty Acids in the Body
Before talking about the important role of fatty acids, let’s first get a better idea of its structure as it’ll help us to understand its role much better.
A. Fatty Acid Structure
Similar to other molecules, its name already gives us an idea of its structure. Fatty acids have 2 main components: a carboxylic acid group and a hydrocarbon chain.
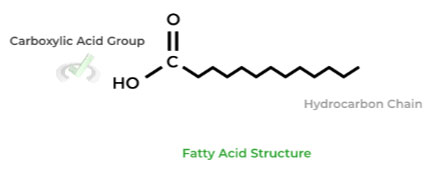
Furthermore, fatty acids can further be classified into saturated or unsaturated which describes the presence of double bonds in the hydrocarbon chain.
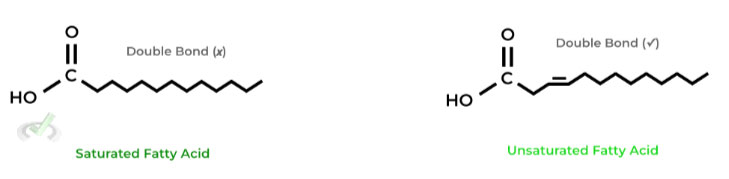
As shown above, saturated fatty acids lack a double bond and unsaturated fatty acids contain a double bond. These become important when discussing their role in the cell membrane!
Additionally, monounsaturated and polyunsaturated describe the amount of double bonds present in an unsaturated fatty acid, with the latter having multiple.I. Cis v.s. Trans Orientation
Unsaturated fatty acids can be also categorized by the orientation of the chain around the double bond!
A cis orientation occurs when the hydrogen atoms are on the SAME side while a trans orientation occurs when the atoms are on OPPOSITE sides.

Regardless of the orientation, the presence of a double bond in unsaturated fatty acids will induce a kink, which will be important later in the article!
II. Omega Nomenclature
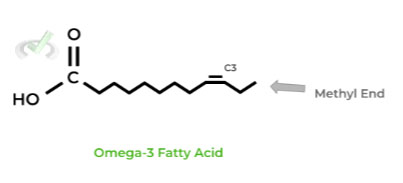
You may have heard of this term in things like omega-3 and omega-6 fatty acids, but they’re actually just nomenclature systems describing the location of the double bond!
The number refers to the carbon where the first double bond is located coming from the METHYL end. Make sure to count from this end and not the carboxylic acid end!
B. Role of Fatty Acids
In the cell, fatty acids serve 3 main roles: 1) cell membrane structure, 2) membrane fluidity, and 3) energy storage. Let’s take a closer look at each one of them!
I. Cell Membrane Structure
Recall that the cell membrane is composed of a phospholipid bilayer, with the most common component being a glycerophospholipid, which uses a glycerol backbone connected to 2 fatty acid tails and a phosphate group.
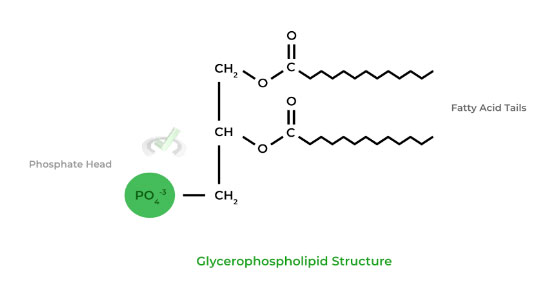
Also recall that the hydrophilic, phosphate head is facing the extracellular and/or cytosolic sides while the fatty acid tails compose the hydrophobic core.
II. Membrane Fluidity
Aside from just being a component of the cell membrane, fatty acids also aid in regulating the fluidity of the membrane.
Generally, saturated fatty acids in the membrane DECREASES membrane fluidity while unsaturated fatty acids INCREASES it.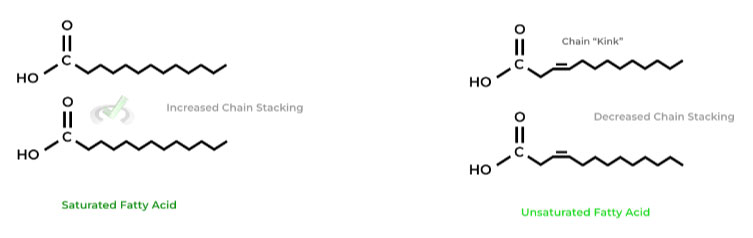
This is due to the chain “kink” that emerges due to the double bond of unsaturated fatty acids, which prevents the stacking of the hydrocarbon chains, preventing their hydrophobic interaction and increasing membrane fluidity!
Saturated fatty acids don’t have a kink and have a more “linear” arrangement of their chains, allowing for the hydrophobic stacking, decreasing fluidity.III. Energy Storage
Finally, fatty acids also can be stored as an energy source within the cell! We’ll go over the actual pathways in another article but for now, we’ll cover its structure.
Fatty acids are stored as energy as triacylglycerols, also commonly known as triglycerides.
It’s very similar to a glycerophospholipid, except that the phosphate head is replaced by another fatty acid chain.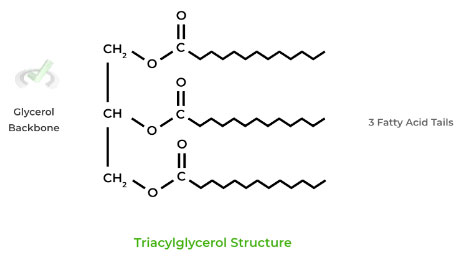
III. Bridge/Overlap
How are triglycerides and even glycerophospholipids synthesized? Their synthesis is centered on a crucial organic chemistry reaction: esterification. Let’s briefly review this mechanism!
I. Esterification
Recall that esterification is a type of nucleophilic substitution where an alcohol (-OH) will substitute the hydroxyl group of a carboxylic acid (-COOH), generating an ester (-COOR).
In the case of triglycerides, the alcohol groups on the glycerol molecule undergo esterification 3 times for each of the 3 alcohols by attacking 3 fatty acids as shown below!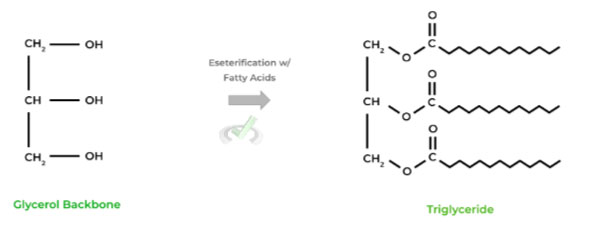
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Fatty Acid Structure
All fatty acids are composed of a carboxylic acid group and a hydrocarbon chain. Furthermore, they can be classified into saturated or unsaturated fatty acids describing the lack or presence of double bonds, respectively.
Additionally, unsaturated fatty acids can be classified into monounsaturated or polyunsaturated describing the amount of double bonds present, with the latter having multiple.I. Cis v.s. Trans Orientation
Unsaturated fatty acids can also differ in the orientation of the hydrocarbon chain around the double bond!
A cis orientation occurs when the hydrogen atoms are on the SAME side while a trans orientation occurs when the atoms are on OPPOSITE sides.II. Omega Nomenclature
This is one popular way fatty acids can be described where the number refers to the carbon where the first double bond is located when counting first from the METHYL end! Remember not to confuse this with the carboxylic acid end!
B. Role of Fatty Acids
Fatty acids serve 3 crucial cellular roles: 1) cell membrane structure, 2) membrane fluidity, and 3) energy storage.
I. Cell Membrane Structure
Fatty acids will comprise a significant portion of the membrane’s hydrophobic core. As an example, the most common membrane phospholipid is glycerophospholipid, which is a glycerol backbone connected to 2 fatty acid tails and a phosphate group.
The phosphate heads are situated to face the extracellular and/or cytosolic side while the fatty acids compose the hydrophobic core.II. Membrane Fluidity
Fatty acids also have a role in membrane fluidity! In general, saturated fatty acids will DECREASE membrane fluidity while unsaturated fatty acids will INCREASE it.
This is due to the chain kink formed via the double bond in unsaturated fatty acids. The kink prevents hydrophobic stacking of the chains, increasing fluidity.
Saturated fatty acids lack this kink due to a lack of a double bond, which allows for the hydrophobic stacking, decreasing fluidity.III. Energy Storage
Furthermore, fatty acids can also serve as an energy source, where they are stored in cells as triacylglycerols, also commonly known as triglycerides.
It has a very similar structure to a glycerophospholipid, except another fatty acid chain is substituted for the phosphate head group.V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
Given the fatty acid shown below, which of the following is the best way to describe its structure?
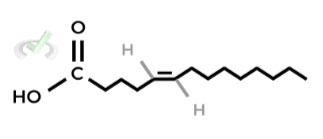
A. Cis, Omega-9 Fatty Acid
B. Cis, Omega-5 Fatty Acid
C. Trans, Omega-9 Fatty Acid
D. Trans, Omega-5 Fatty Acid
Ans. C
Remember, when doing omega nomenclature, start counting from the methyl end! From the methyl end, the first carbon that contains the double bond is the C9 carbon.
In addition, the hydrogen atoms around the double bond are on the OPPOSITE sides, giving it a trans orientation.
Sample Practice Question 2
Integral proteins are proteins which span the entire membrane which contains a transmembrane domain which interacts with the fatty acids of the hydrophobic core. Which of the following amino acids pairs would NOT be found present on the surface of the transmembrane domain?
A. L, V
B. A, G
C. C, E
D. W, F
Ans. C
Recall that these amino amino acids are hydrophilic and won’t form favorable interactions with the fatty acid hydrophobic interior.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
