I. What is the Metabolism of Proteins?
Most often in the MCAT, you’ll associate metabolism with carbohydrates and to a lesser extent lipids. Even to a lesser extent, you can associate metabolism with proteins as they are scarcely utilized as an energy source in the human body!
This is due to the importance of proteins in the body in terms of both structure and function which makes sense logically: proteins constitute a huge structural portion for a variety of structures like skeletal and cardiac muscle. Utilizing protein as an energy source could cause defects in these muscles!
Nevertheless, in times of grave starvation, proteins can and will be utilized as a last means energy source. Similar to some of the other articles in this section, there’s no need to memorize a lot of details as we’ll give you only what’s necessary for the MCAT exam!II. Components of Protein Metabolism
Similar to lipids, proteins begin their metabolism during digestion from which then the smaller components can enter into cellular, biochemical pathways. Let’s delve into them a little bit!
A. Protein Digestion
Just like all food particles, dietary proteins must be broken down into smaller peptides and individual amino acids in the stomach to aid in their absorption.
Specialized enzymes called peptidases catalyze the proteolytic cleavage of proteins to their smaller components, in addition to the acidic pH of the stomach.
Some common peptidases, also called proteases, include pepsin, trypsin, and carboxypeptidase as listed above and as mentioned in our digestive system articles!
Afterwards, these amino acids are absorbed in the small intestine and distributed accordingly throughout the body.
B. Cellular, Biochemical Pathways
As mentioned above, the body can also catabolise proteins if needed as an energy source via intracellular proteolytic cleavage, which can then enter into the ketogenic and/or gluconeogenic pathways depending on the amino acid.
Oftentimes, the amino acids must remove their amino group through deamination before entering into the pathways.
Because of its possible toxicity as a form of ammonia, the amino group is metabolized in the liver to urea, which can be excreted by the kidney.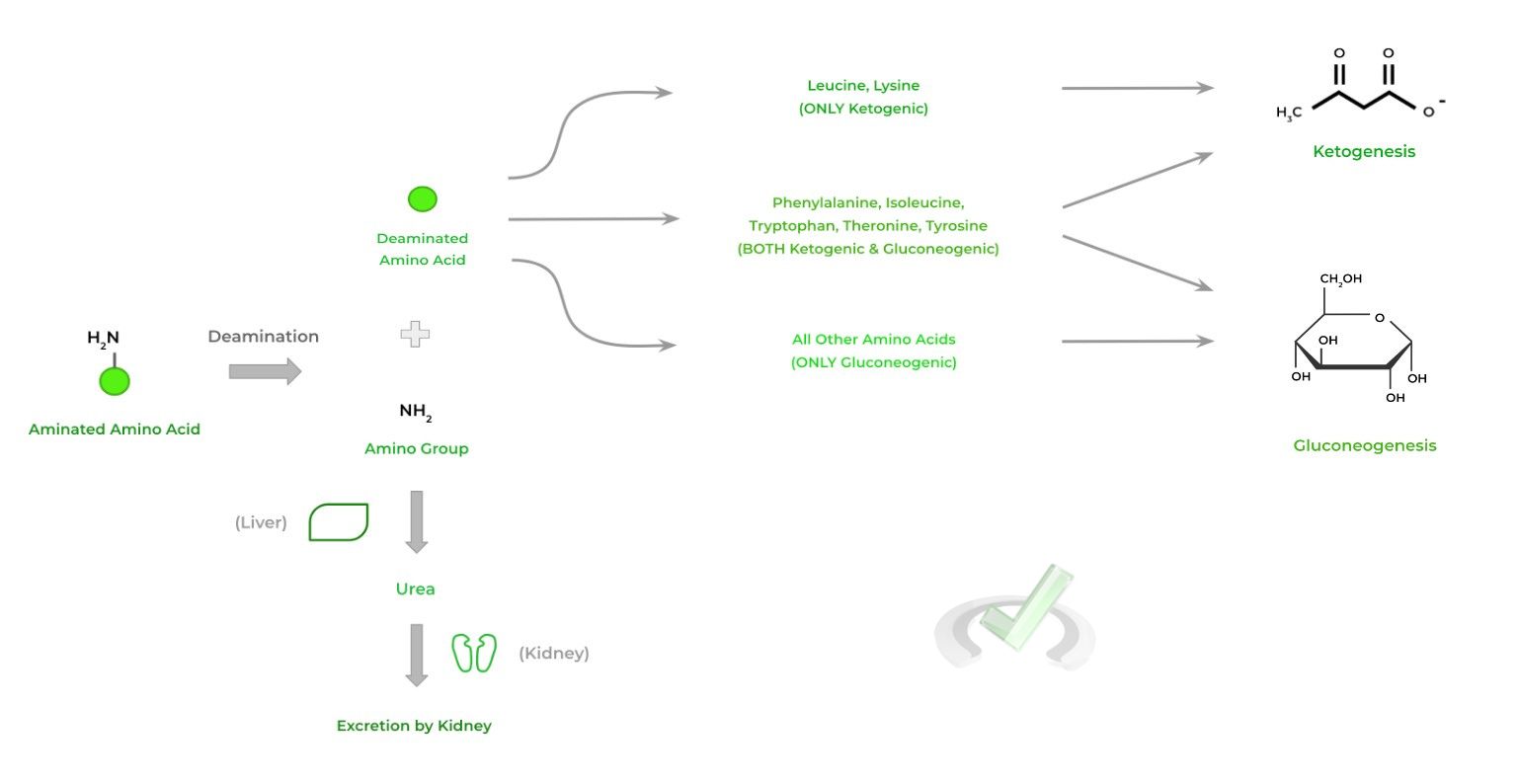
III. Bridge/Overlap
Just like with other enzymes like ATPases and caspases, peptidases catalyze the proteolytic cleavage of proteins via hydrolysis! Let’s take a quick look and review of the mechanism!
I. Peptidase Catalyzed Protein Hydrolysis Mechanism
In its simplest form, the reaction is an example of a nucleophilic substitution reaction, where the H20 molecule acts as the nucleophile attacking the electrophilic carbonyl carbon.
Note also that this is essentially the reverse process of dehydration synthesis, as instead of a water molecule being generated, we use a water molecule to break down a molecule.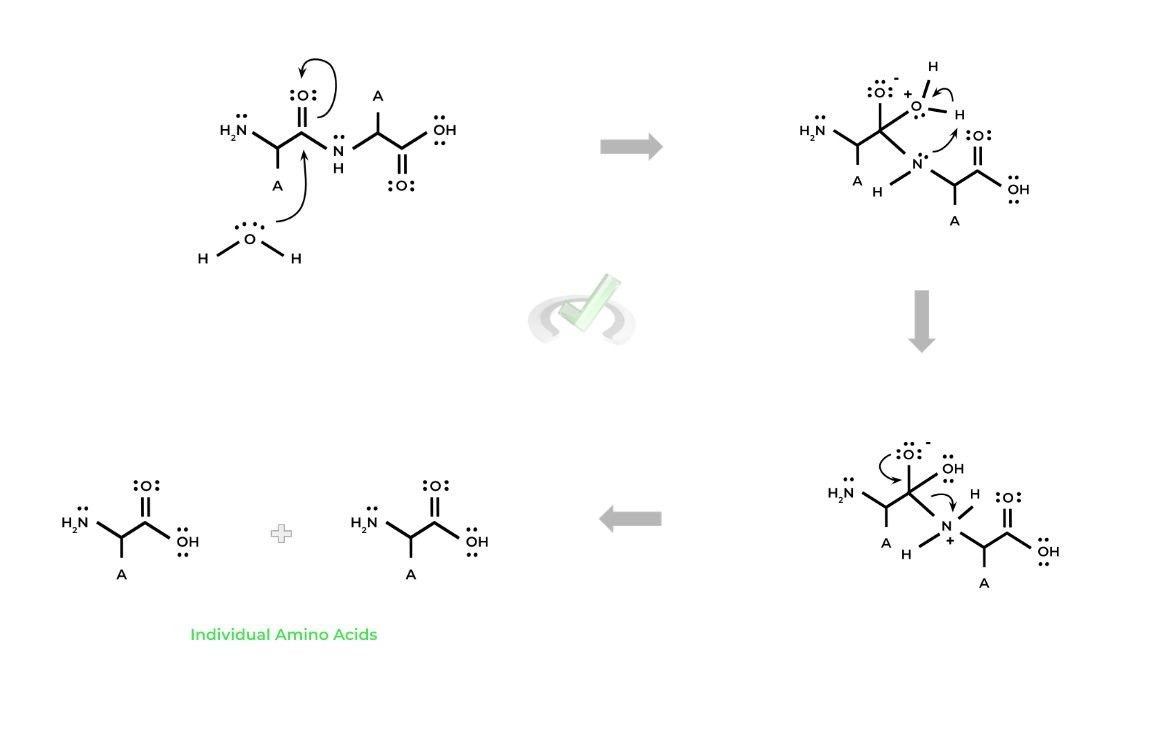
IV. Wrap Up/Key Terms
Let’s take this time to wrap up & concisely summarize what we covered above in the article!
A. Protein Digestion
Just like other food particles, dietary proteins are broken down into smaller peptides and amino acids to aid in absorption, accomplished by peptidases and the highly acidic pH in the stomach.
B. Cellular, Biochemical Pathways
In severe states of starvation, proteins can actually be mobilized as an energy source! After intracellular proteolytic cleavage and deamination, the amino acids can enter into either ketogenic and or/ gluconeogenic pathways, depending on the amino acids.
Leucine and lysine (LL) are ONLY ketogenic while phenylalanine, isoleucine, tryptophan, threonine, and tyrosine (PITTT) are BOTH ketogenic and gluconeogenic. The remaining amino acids are ONLY gluconeogenic.
V. Practice
Take a look at these practice questions to see and solidify your understanding!
Sample Practice Question 1
When pepsin enacts its proteolytic hydrolysis on proteins, what type of bond is broken?
A. Ester
B. Phosphoanhydride
C. Peptide
D. Phosphodiester
Ans. C
Recall that proteins are basically a series of amino acids connected by a peptide bond, which is also called an amide bond which is synthesized via the nucleophilic attack of the nucleophilic amine group on the electrophilic carbonyl carbon.
Sample Practice Question 2
If a protein with the sequence D-S-C-H is cleaved leaving only the individual amino acids which need to be used for energy, which of the following is true?
A. The production of acetoacetate will increase
B. The production of glucose will increase
C. The production of both glucose and acetoacetate will increase
D. The production of glucose will decrease
Ans. B
Recall that only leucine, lysine, phenylalanine, isoleucine, threonine, tryptophan, and tyrosine are capable of producing ketones, so any answer which produces ketones is incorrect.
All the remaining amino acids from the ones listed above are ONLY gluconeogenic and in times when energy is needed, these remaining amino acids will enter into gluconeogenic pathways to increase glucose production to be used as an energy source.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
