Atoms make up everything in this world. For elements, an atom is its smallest unit. The atoms of each element differ from each other. This difference is characterized by the difference in the number of subatomic particles. Subatomic particles are particles that are much smaller than the atom. They are what makes the atom unique.
We’ve previously touched on these subatomic particles: the proton, neutron, and electrons.
In this article, we’re going to dive a little deeper into each subatomic particle and explore their properties and what they contribute to the atom.
I. The atomic model
Let’s first discuss the atomic model. The most widely accepted atomic model is the electron cloud model. As the name suggests, this model acknowledges the presence of an electron cloud.
This electron cloud is a cloud or space where electrons are likely to be found. While the electron cloud shows us the most likely position of the electron, the specific area where these electrons might be found is called an orbital.
The nucleus of an atom is the core of an atom. The electron cloud surrounds the proton and neutron (collectively called nucleons).

II. Protons and neutrons
Protons = Protons have a positive nuclear charge. They were discovered in 1919 by Ernest Rutherford in a gold foil experiment. He bombarded a gold foil with alpha (positively charged) particles. He noticed that some light deflected, and others passed through and shone at different angles. This led him to believe that when some of the light deflected, the positive alpha particles hit something that had the same charge. The number of protons is also known as the atomic number that describes an element’s position in the periodic table of elements.
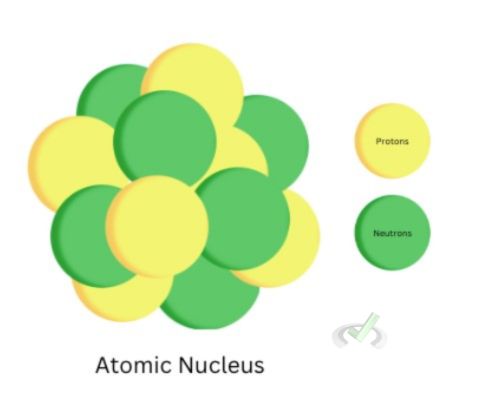
A proton is a positively charged subatomic particle. It has a mass of 1.672510-24 grams which is roughly 1840 times the mass of an electron and a +1 charge equivalent to 1.60210-19coulombs.
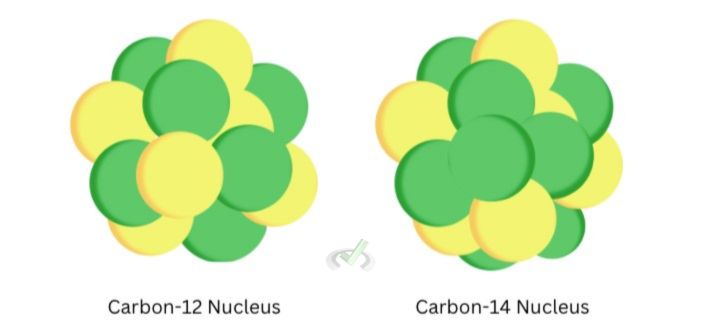
Neutrons are electrically neutral particles that are slightly heavier than protons. The protons and neutrons make up the atomic nucleus. The number of neutrons can vary. Elements that exist with a different number of neutrons are known as isotopes. While protons characterize an element, neutrons do not need to have a set number for a particular element. Carbon-12 is an isotope with six protons and neutrons, another isotope, Carbon-14, also has six protons, but has eight neutrons.
The sum of the number of protons and neutrons represents the atomic mass. This is why carbon-14 is named as such–it has six protons plus eight neutrons.
III. Electrons
Electrons are negatively charged subatomic particles. They move around the nucleus in the electron cloud space. These subatomic particles influence most of the properties of an atom. They are also the only subatomic particles involved in chemical reactions. Since electrons revolve in orbitals, their distribution in the electron cloud influences chemical behavior. They have a mass of 9.109 x 10⁻²⁸ grams and a charge of -1 or 1.602 x 10⁻¹⁹ coulombs.
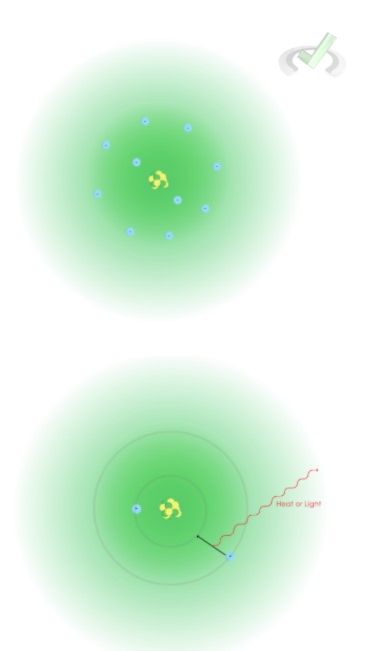
Chemical behavior is highly influenced by the movement of electrons around the nucleus. De-excitation, for example, is a process that occurs when an electron absorbs energy and goes to a higher energy level. This is followed by de-excitation or the release of energy when the electron falls back to its original energy level. When de-excitation happens, energy is released in the form of heat or light. Electrons are directly involved in chemical bonding due to their presence in the outer shell of an atom.
IV. How subatomic particles relate to each other
In an atom, the electrons move around a space surrounding the nucleus containing protons and neutrons. This movement is due to the electrostatic force between the positively charged nucleus and the negatively charged electron. Even though electrons are attracted to this positive nucleus, it does not crash into the nucleus as one might think.
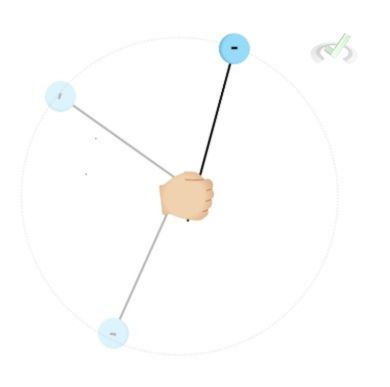
Subatomic particles like to be stable. The attraction between the electron and the nucleus keeps the electrons in orbit around the nucleus. The process is similar to spinning a ball tied to a string. This force keeping the ball tied to a string is the attractive force. What keeps these two separated is their electrostatic energy that allows electrons to have energy to keep moving.
V. Conclusion
Atoms make up everything in this world. An atom of one element differs from another--this is characterized by the atomic number that is equal to the number of protons. Protons are positively charged subatomic particles that can be found in the nucleus of an atom along with a neutron. Neutrons are electrically neutral. The number of protons and neutrons determines the atomic number of an atom. The number of neutrons may vary. When elements exist in varying numbers of neutrons, these elements are known as isotopes. Electrons are negatively charged and are very light. They move around the nucleus and they characterize the chemical properties of a molecule.
VI. Key Terms
- Electrons - Negatively charged subatomic particles.
- Neutrons - Electrically neutral subatomic particles.
- Protons - Positively charged subatomic particles.
VII. Practice Questions
Sample Practice Question 1
Which of the following is FALSE?
A. The number of electrons identifies an element.
B. The atomic mass is equal to the sum of the number of protons and neutrons.
C. The Electrostatic force allows electrons to move around the nucleus.
D. Electrons are involved in chemical bonding.
Ans. A
Sample Practice Question 2
Which is the correct arrangement of the masses of the subatomic particles from heaviest to lightest?
A. Neutron > Electron > Proton
B. Neutron > Proton > Electron
C. Proton > Neutron > Electron
D. roton > Electron > Neutron
Ans. B







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
