In the previous article on subatomic particles, we discussed the role of subatomic particles and how they characterize an element. We figured that the number of protons in an atom identifies the element and the sum of protons and neutrons makes for its atomic mass. We also learned that while atoms of the same element will always have the same number of protons, the number of neutrons for the same element can vary without changing the element. Elements of the same kind with different atomic masses are known as isotopes.
It’s a lot to take in. However, we can take this one step at a time by clarifying the differences between the most commonly misused terms.
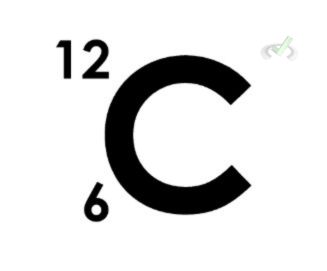
Let’s first make it clear that we denote elements and their respective atomic masses and atomic numbers by the following naming convention:
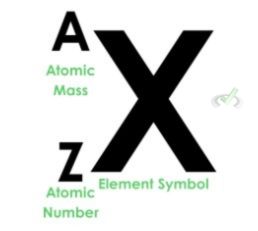
This way, we can differentiate between the digits we use to describe an element. A carbon atom that has an atomic mass of 14 and an atomic number of 6 will have the following notation:
I. Atomic Weight vs. Atomic Mass
When we use atomic mass, we usually interchange this with what we know as mass number. However, atomic mass is an entirely different concept. Atomic mass is also known as isotopic mass. By the name itself, atomic mass is the specific mass of a certain isotope. Silicon-28 is an isotope of silicon that has an atomic mass of 27.976 atomic mass units (amu), this is different from the atomic mass of silicon-30 which is 29.973. Atomic mass refers to the specific mass of an isotope, it does not account for an isotope’s abundance.
The atomic weight of an atom is the average of all the masses of naturally occurring isotopes. You might wonder why this must be the case. Since the same elements can exist having different mass numbers, we must account for their abundance in the natural world.
The concept is simple: if we want to get the average masses of strawberries, blueberries, and raspberries in a basket, we have to account for the amount of each berry and their individual weights to get their average mass. This way, we have a better distribution of masses and we’re accounting for the abundance of each separate berry.
Let’s try this out with the isotopes of silicon.
Isotope | abundance | atomic mass | abundance x atomic mass |
|---|---|---|---|
Silicon-28 | 92.23% | 27.976 | 28.8022 |
Silicon-29 | 4.67% | 28.975 | 1.3531 |
Silicon-30 | 3.10% | 29.973 | 0.9292 |
Atomic Weight (sum) | 28.0845 | ||
The atomic weight we get is 28.0845 which is roughly equal to the atomic weight of silicon. Atomic weights are practically unitless. We can use atomic mass units, but it’s a relative measure of how much an element weighs.
II. Atomic Number vs. Mass number
We previously discussed subatomic particles and found that the number of protons is the atomic number of an element. We also defined mass number as the sum of the amount of protons and neutrons in an atom.
The atomic number, Z, characterizes an element. Silicon will always have 14 protons, silicon-28, silicon-29, and silicon-30 have 14 protons. These isotopes, however, will differ in the number of neutrons. Silicon 28 has 14 neutrons, silicon-29 has 15, and silicon-30 has 16 neutrons. Isotopes are elements of the same kind that bear different mass numbers.
Mass number, on the other hand, is characterized as the sum of protons and neutrons in an element. Isotopes will always have the same atomic number but they will also have different mass numbers since isotopes have different numbers of protons.
Isotope | atomic number | number of protons and neutrons | mass number |
|---|---|---|---|
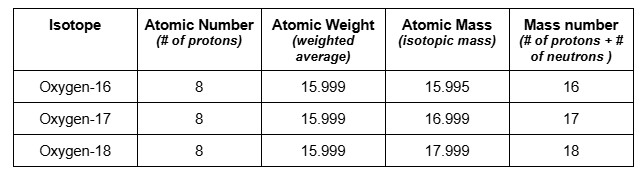
Oxygen-16 | 8 | 8 protons; 8 neutrons | 16 |
Oxygen-17 | 8 | 8 protons; 9 neutrons | 17 |
Oxygen-18 | 8 | 8 protons; 10 neutrons | 18 |
III. Atomic Weight vs. Atomic Mass vs. Mass Number vs. Atomic Number
To fully understand the differences between these four terms, here are a few examples you can refer to.
1. Silicon
2. Oxygen
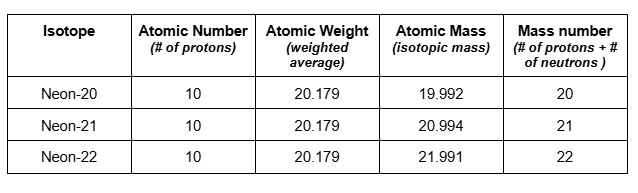
3. Neon
IV. Conclusion
We use a lot of quantities to describe an element. Among these are the numbers related to the mass or weight of an aton. Atomic mass, atomic number, atomic weight, and mass number are quantities related to how many subatomic particles are inside an atom. Atomic mass refers to the isotopic mass, it is a value that is unique to an isotope of an element. Isotopes of the same element will always have different masses.
On the other hand, atomic weight is the weighted average of all naturally occurring isotopes in an element. We calculate atomic weight by considering the weight and the abundance of each isotope. The mass number is a quantity that describes the total number of protons and neutrons in an isotope. Meanwhile, the atomic number refers to the number of protons in an element. Isotopes of an element will always have the same atomic number and weight for that specific element, but different atomic mass and mass number.
V. Key Terms
- Atomic Mass - the mass of an isotope.
- Atomic Number - the number of protons in an element that identifies the element.
- Atomic Weight - the average weight of all naturally occurring isotopes.
- Mass number - the sum of the number of protons and neutrons in the nucleus.
VI. Practice Questions
Sample Practice Question 1
Calculate the atomic weight of nitrogen given the following abundance and atomic mass of its isotopes:
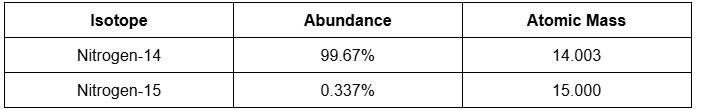
A. 15.502
B. 14.000
C. 15.025
D. 14.007
Ans. D
Sample Practice Question 2
Which of these statements is FALSE?
A. The atomic number is equal to the number of protons
B. The mass number is the total number of nucleons in the nucleus.
C. Atomic weight varies among isotopes.
D. Atomic mass is the isotopic mass.
Ans. C







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
