Electron Configuration is how we can represent how an electron is distributed in atomic orbitals. We use electron configuration to distinguish an element and determine how the electrons are arranged. We do this using a notation that represents which energy level is filled up, the type of orbital, and the number of electrons in that specific orbital.
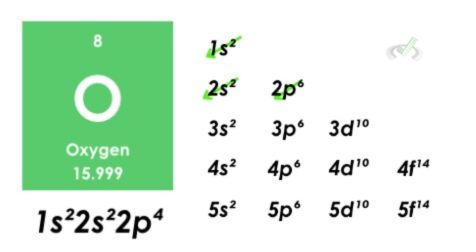
For example, let’s look at the electron configuration of oxygen.
- Oxygen has 8 electrons.
- The superscripts represent the number of electrons in each orbital. There are 2 electrons on two s orbitals and 4 electrons on one p orbital. The sum of these electrons is equal to 8.
- 1s orbital gets filled first, then 2s, and lastly the 2p orbital. Since it can hold up to six electrons and there are four electrons left after filling up two s orbitals, the final orbital will be denoted as 2p⁴
Let's explore how this works and the principles that rule electron configuration.
I. Aufbau Principle
We've briefly summarized these concepts in the article on quantum numbers. Since quantum numbers are concerned with describing the probable position of an electron within an atom, the idea of electron configuration is quite related. For one, we can use an atom's electron configuration to determine an electron's quantum number. Nevertheless, these concepts follow the same principle. One of which is the Aufbau principle.
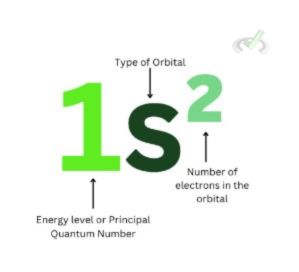
This states that when we put electrons in orbitals, we fill out the lowest energy level first. This is why in our Aufbau principle diagram, 1s orbital comes first. Once it's filled with two electrons, 2s orbital comes next, 2p with six electrons, 3p with two, and so on.
Electrons like to be stable. We're putting electrons in the most stable energy and electron distribution by filling up the lowest energy level. Suppose an electron is at 5f and no other electrons are filling in for the s, p, and d orbitals. In that case, that's a highly unstable and improbable atom.
This is also why, in orbital diagrams, we see such order being followed in assigning electron spin. We want to fill up electrons at the lowest energy level before moving on to higher energy levels.
II. Pauli Exclusion Principle
Pauli's exclusion principle states that no two electrons can share the same quantum numbers. When we look at our orbital diagram for oxygen, notice that each electron has its own blocks. The two electrons sharing the orbital will always have opposite spins. This allows electrons to have unique quantum numbers.
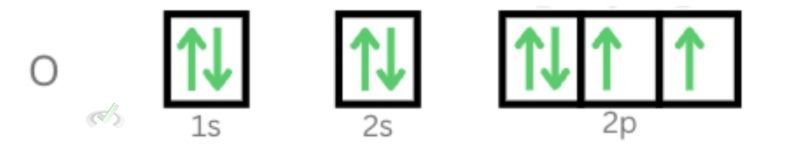
One might argue that electrons with 1s orbital will have the same quantum numbers for oxygen since they share the same orbital with n = 1, l = 0, and m = 0. The difference here is that these electrons will always differ when it comes to electron spin. One will go clockwise (+½), and the other will go counterclockwise (-½).
III. Hund’s Rule
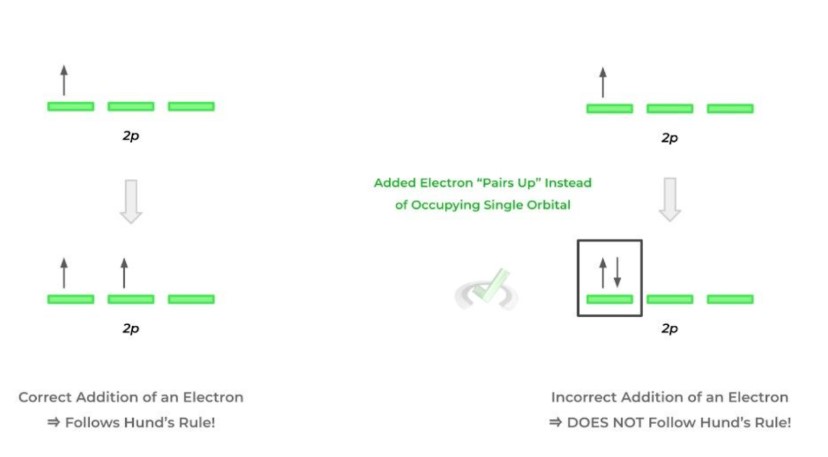
Hund's rule states that when we assign electrons in the same subshell, electrons must be individually put in orbitals before getting paired up. This rule matters because it minimizes the repulsion between electrons. Having electrons in the same spin put in different orbitals will be more stable than having electrons with opposite spins in one orbital. Since we want to achieve stability, we keep things less energy-consuming and as less repulsive as possible.
IV. Other Examples
Let’s try to do electron configuration using what we know so far.
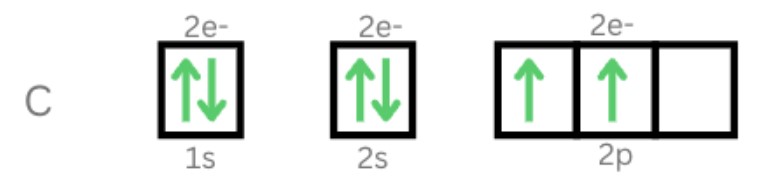
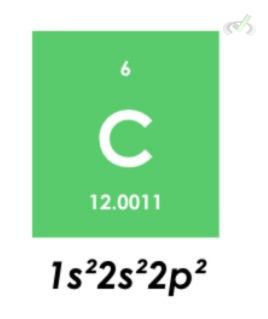
1. Carbon Aufbau Principle
Carbon has six electrons. Since the lowest energy level comes first, we must fill out the 1s orbital with two electrons. We still have four electrons, and the two electrons will fill out the 2s orbital first. The last two will fill out the last orbital with two electrons.
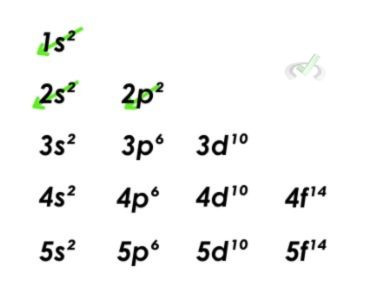
Pauli’s Exclusion Principle
Here, the 2p orbital will fill out two of the six possible electrons that could be taken up by electrons. All the orbitals filled out had opposite spins except the ones that had not been paired up yet. The 2p orbitals are filled up individually instead of being put in one orbital with the same spin.
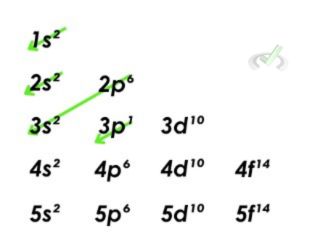
2. Aluminum
- Aluminum has 13 electrons. Let's look at our Aufbau principle diagram. We can see that the orbitals are filled out until the 3p orbital, where the thirteenth electron lies. This means that the electron configuration for aluminum is 1s²2s²2p⁶3s²3p¹.
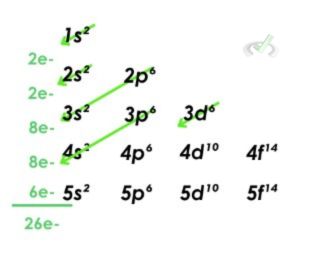
3. Iron
- Iron has 26 electrons. All orbitals are filled out until the 3d orbital with 6 electrons. Therefore, the electron configuration for Iron is 1s²2s²2p⁶3s²3p⁶4s²3d⁶.
- This electron configuration can be simplified using the electron configuration of a noble gas. Noble gases are stable, and their orbitals are filled out completely. We can then choose the noble gas closest to Iron, Argon. Argon has an electron configuration of 1s²2s²2p⁶3s²3p⁶. We can then rewrite the
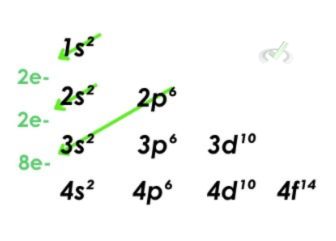
4. Magnesium ion (Mg²⁺)
- Let's first look at the electron configuration of the Mg atom. The orbitals will fill out until the 3s orbital. A neutral Magnesium atom will have an electron configuration of 1s22s22p63s2. A positive ion has fewer electrons than a neutral atom. We can remove two electrons from the magnesium ion configuration and have an electron configuration of 1s²2s²2p⁶ for the magnesium ion.
V. Conclusion
Electron configurations allow us to see how electrons are distributed in an atom. This notation allows us to understand where electrons might be in an atom. This notation is governed by principles such as the Aufbau principle, which states that electrons will fill out the lowest energy level first. Pauli's exclusion principle dictates that no two electrons will have the same quantum number. Hund's rule states that every orbital in a sublevel will be singly filled before getting paired up. These rules allow us to understand electrons and how their position and behavior contribute to their properties.
VI. Key Terms
- Aufbau Principle - Electrons will first occupy the orbitals with the lowest energy levels during electron distribution.
- Electron Configuration - A way of showing how electrons are distributed around the nucleus.
- Hund's Rule - Electrons in the same orbital will be filled out individually before being paired with an electron.
- Pauli's Exclusion Principle - No two electrons of the same atom will have the same set of quantum numbers.
VII. Practice Questions
Sample Practice Question 1
Determine the quantum number of the fourth electron in Neon, whose electron configuration is 1s²2s²2p⁶
A. (2, 0, 0, -1/2)
B. (1, 0, 0, 1/2)
C. (2, 0, 0, 1/2)
D. (1, 0, 0, -1/2)
Ans. A
Sample Practice Question 2
Which of the following electron configurations is NOT correct?
A. Aluminum: [Ne]3s²3p¹
B. Neon: [He]2s²2p⁶
C. Phosphorus: [Ne]3s³2p⁶
D. Lithium: [He]2s¹
Ans. C







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
