We have briefly discussed bonds in previous articles, and two common terms—ionic and covalent bonds — must already be familiar to you.
Chemical bonding is simple. When two elements form a bond, they create a compound made up of two or more elements. Some elements can bond with each other, and other elements don’t even form a bond at all!
In this article, we’ll explore chemical bonds a little more to better understand the whole process and distinguish between covalent and ionic bonds. We’ll also discuss other less common types of bonds!
I. Ionic bonds
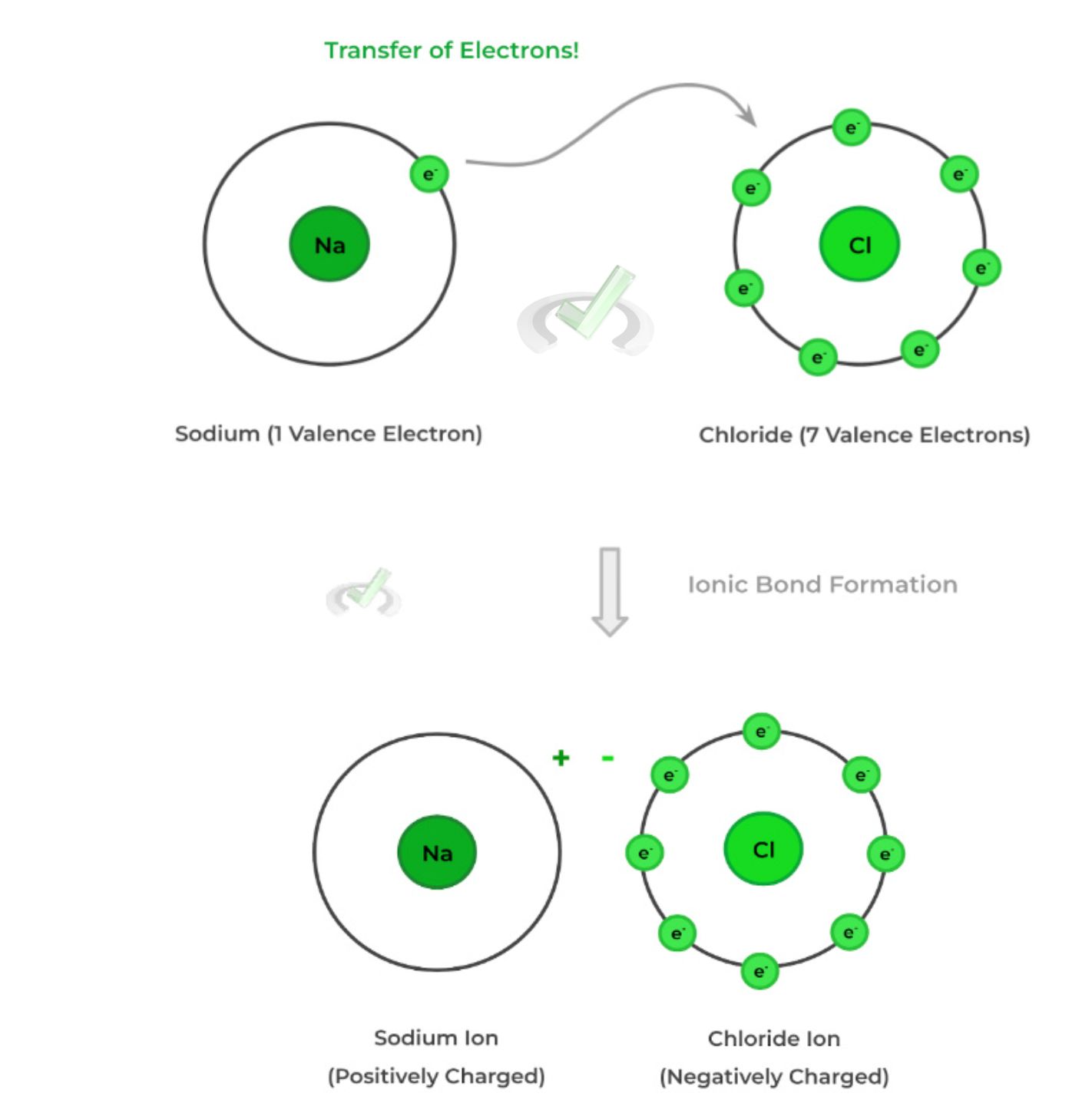
Ionic bonds form through the transfer of electrons. The bond happens only between metals and nonmetals.
In this process, a metal donates an electron to a nonmetal. When the metal loses an electron, it becomes positively charged. As the nonmetal gains an electron, it becomes negatively charged.
In the image to the right, sodium is a metal that donates one electron to chloride. Sodium gains a positive charge after donating an electron, and chlorine gains a negative charge as it receives an electron.
The transfer of electrons is due to the electrostatic interaction between the metal and nonmetal. Sodium chloride (NaCl) is held by electrostatic attraction since their charges attract each other, forming a stable ionic bond.
II. Covalent bond
Covalent bonds are formed between nonmetals. Unlike ionic bonds, where electrons are transferred from a metal to a nonmetal, covalent bonds form by sharing electrons. In this process, an element does not lose an electron–it merely shares an electron from a different element.
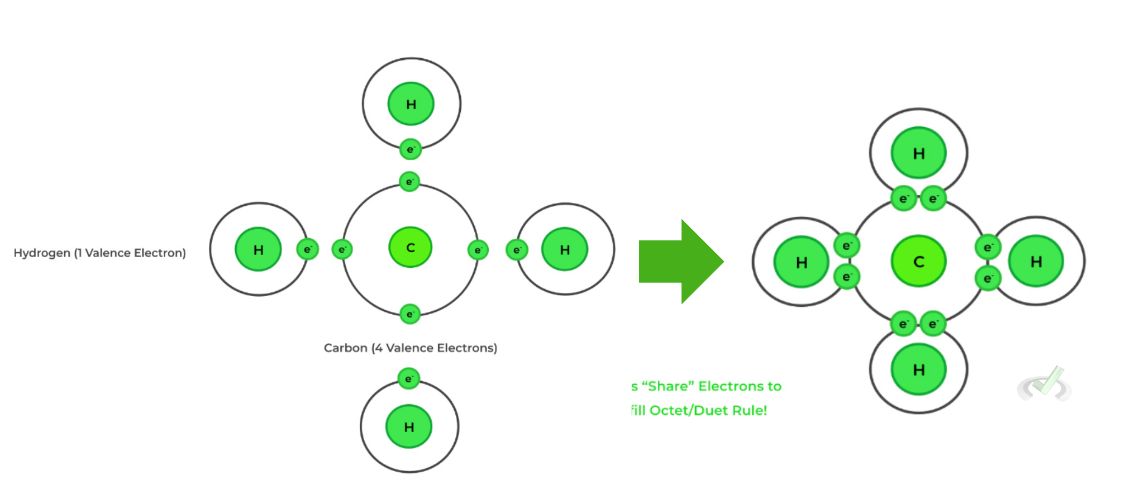
In the example above, carbon and hydrogen are nonmetals. When they bond, both elements retain their original electrons but share the same orbital to complete the octet.
Octet Rule
This concept may or may not be familiar to you, but the octet rule is a rule of thumb for chemical bonds. This rule states that the valence shell of bonding atoms should have eight electrons. In the example above, CH₄, or methane, forms a bond with four hydrogen atoms.
Carbon initially had four valence electrons. There must be a total of eight electrons surrounding the carbon atom to satisfy the octet rule. This is why carbon is bonded to four hydrogen atoms–it needs four additional electrons to complete its outer shell. As mentioned before, atoms want a completely filled valence shell and for bonding electrons, this is satisfied by having eight electrons surrounding a central atom.
Of course, every rule has its exceptions. For instance, hydrogen, with only one electron, forms a stable diatomic molecule with just two electrons, known as the duet rule. These exceptions add depth to our understanding of chemical bonding.
We’ll talk more on the octet rule in the next articles.
III. Other Forces
Covalent and ionic bonds are more seen in chemistry. However, there are other interactions that happen between molecules as a result of attractive forces. These are hydrogen bonds, Van der Waals forces, and metallic bonds.
A. Hydrogen Bonds
Hydrogen bonds are brought about by attractive forces in a hydrogen atom covalently bonded to an electronegative atom such as oxygen. This type of attractive force can be seen in water molecules. Since oxygen is highly electronegative, it attracts the hydrogen covalently bonded to another oxygen molecule.
B. Van der Waals forces
Van der Waals forces are forces that occur between molecules that are close to each other. These are much weaker than the bonds holding molecules together.
A molecule can momentarily have a negative pole and a positive pole. The negative pole will have a lot of electrons, and the positive end will not. When a lot of these molecules are present, the negative pole of one molecule will be attracted to the positive pole of another.
C. Metallic Bonds
When we look inside a metal, we see that there is a regular pattern between the atoms, and they usually give up an electron in the outer shell, forming an arranged pattern of positively charged atoms. The electrons that are given up are all shared between them, creating a sea of delocalized electrons.
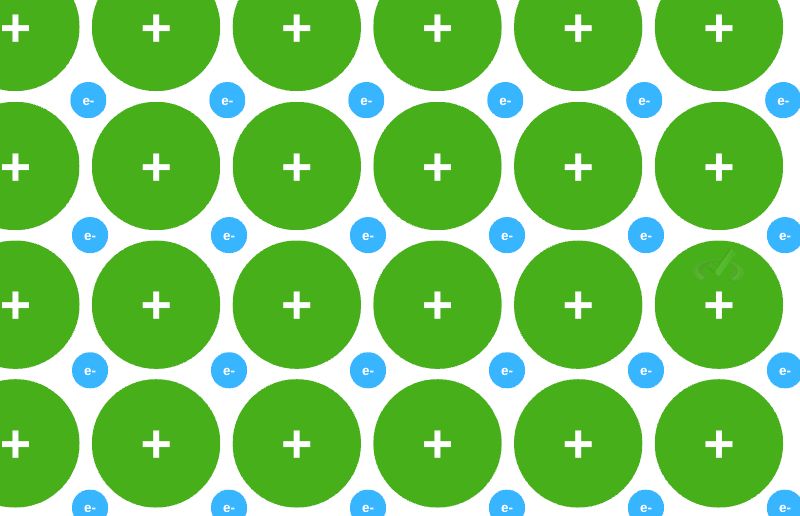
The free electrons will still be held by the surrounding cations. These electrons are held together by the surrounding cations that are also arranged in an orderly manner. This attractive force that keeps the delocalized electrons and the cation is what we call metallic bonding.
IV. Conclusion
The main types of bonding are ionic and covalent bonds. These bonds happen between atoms. Ionic bonds happen between metals and nonmetals through a transfer of electrons. The metal gives its electrons to the nonmetal. As the metal loses an electron, it gains a positive charge, and as the nonmetal gains an electron, it gains a negative charge.
Covalent bonds occur between nonmetals by sharing electrons. To attain a stable bonded atom, a bonding atom tends to have eight electrons, which is also known as the octet rule.
Other forces happen between molecules as a result of attractive forces. These are hydrogen bonds, Van der Waals forces, and metallic bonds.
V. Key Terms
- Covalent bond - A bond that occurs between nonmetals by sharing electrons.
- Hydrogen bond - A bond that happens when an electronegative atom is bonded to hydrogen and attracts another hydrogen bond to another molecule.
- Ionic bond - A bond that forms between metals and nonmetals through the transfer of electrons.
- Metallic bond - An attraction that forms as a result of the attractive force between delocalized electrons and positive cations in metals.
- Van der Waals forces - Attractive forces that occur between the positive side of one molecule and the negative side of another.
V. Key Terms
Sample Practice Question 1
Which of the following statements is NOT TRUE?
A. Lithium oxide (Li₂O) forms through an ionic bond.
B. Lithium donates 2 electrons from its valence electron to form lithium oxide.
C. Carbon dioxide (CO₂) forms through a covalent bond.
D. Two oxygen atoms share their valence electrons to form carbon dioxide.
Ans. B
Sample Practice Question 2
All of the following statements explain chemical bonding except:
A. Hydrogen bond occurs when an electronegative atom attracts hydrogen from another molecule.
B. Van der waals forces occur through intermolecular forces.
C. Metallic bonds occur between alkali metals and alkaline earth metals.
D. The octet rule states that an atom will form bonds in such a way that leaves it with eight valence electrons.
Ans. C
Rationale: Metallic bonds occur between metal atoms, not specifically between alkali metals and alkaline earth metals. Alkali and alkaline earth metals typically form ionic bonds when combined with nonmetals, not metallic bonds.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
