During our discussion of chemical bonds, we discussed that there are forces that occur as a result of the attraction between molecules. This attractive force that forms between molecules is known as an intermolecular force.
These should not be confused with chemical bonds. While we will see the term “bond” occasionally, intermolecular forces do not involve chemical bonding–it merely describes the attractive forces between molecules.
These intermolecular forces exist due to interactions between molecules. We’ve previously discussed polarity, which describes the electron distribution between molecules.
A molecule is nonpolar when the electron distribution between bonding atoms is equal. A polar molecule has an uneven electron distribution between bonding atoms.
I. Van der Waals Forces
Van der Waals forces are attractive forces that result from the attraction among temporary dipoles as a result of fluctuations in electron density. Dipole-dipole interactions, on the other hand, are much stronger and more stable since the molecules we deal with already have opposite poles. In a dipole-dipole interaction, the positive pole of one molecule is attracted to the negative pole of the other.
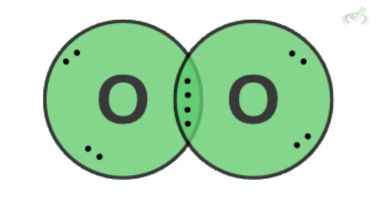
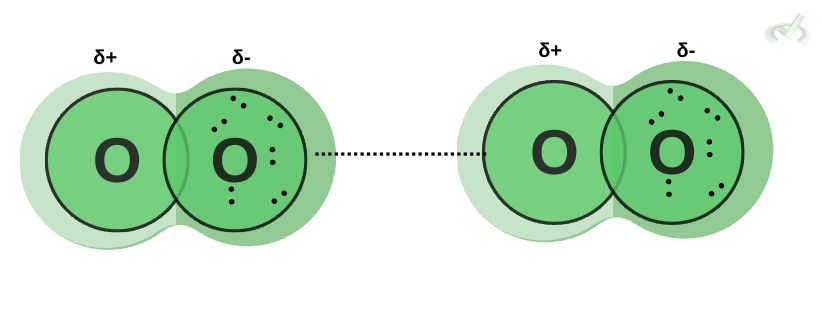
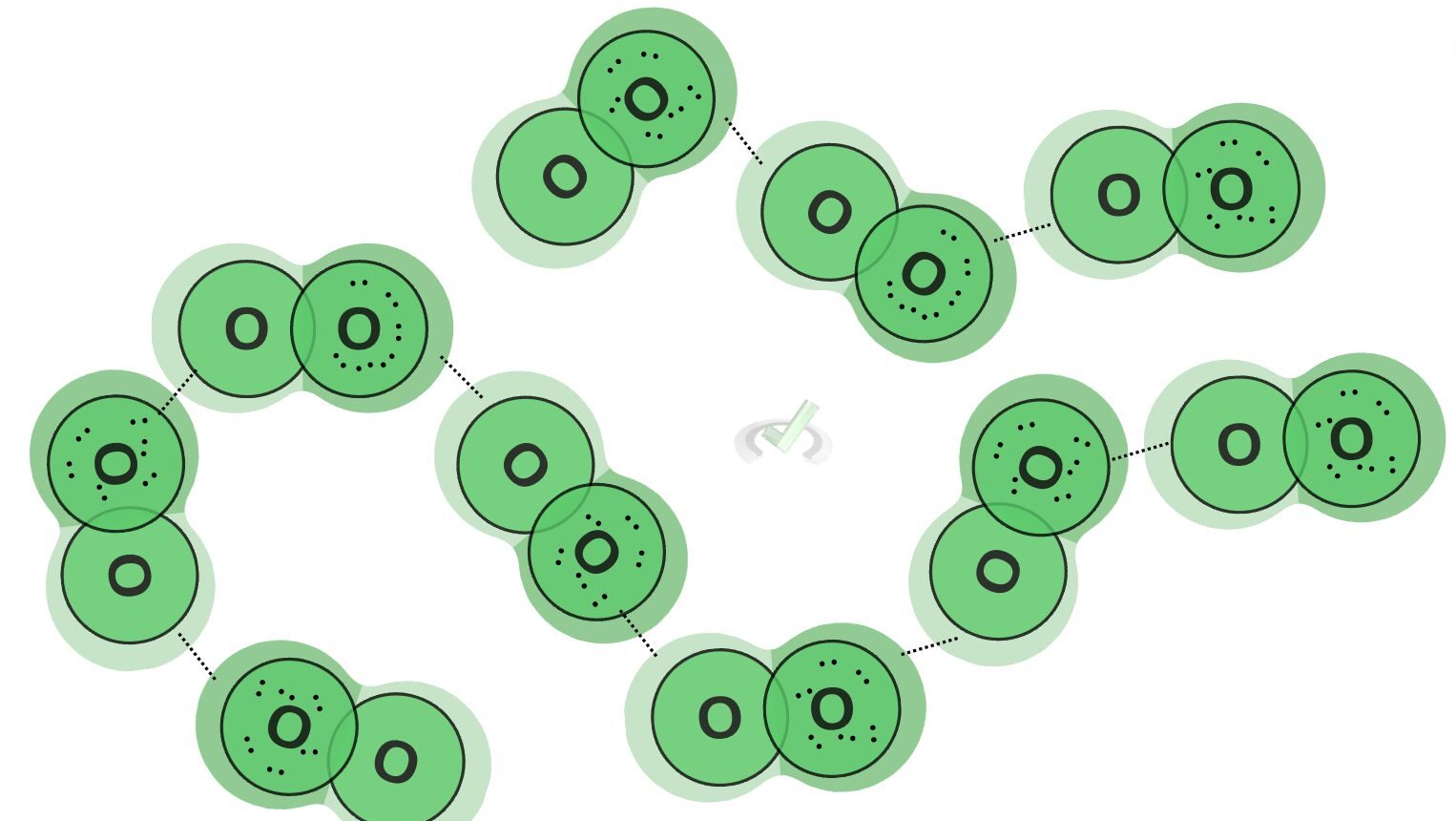
Oxygen is a nonpolar molecule since it has an equal distribution of electrons. However, it can momentarily form temporary dipoles. Since electrons constantly move around a molecule, there are instances where electrons collect or localize on one side. This forms a temporary dipole. This attraction between temporary dipoles is what we call Van der Waals forces.
Van der Waals forces are applicable in many areas of the environment. They influence the ability of geckos to stick to a wall when climbing. The attraction between temporary dipoles gives geckos incredible adhesion to a surface.
II. Dipole-Dipole Interactions
Van der Waals forces are attractive forces that result from the attraction among temporary dipoles as a result of fluctuations in electron density. Dipole-dipole interactions, on the other hand, are much stronger and more stable since the molecules we deal with already have opposite poles. In a dipole-dipole interaction, the positive pole of one molecule is attracted to the negative pole of the other.
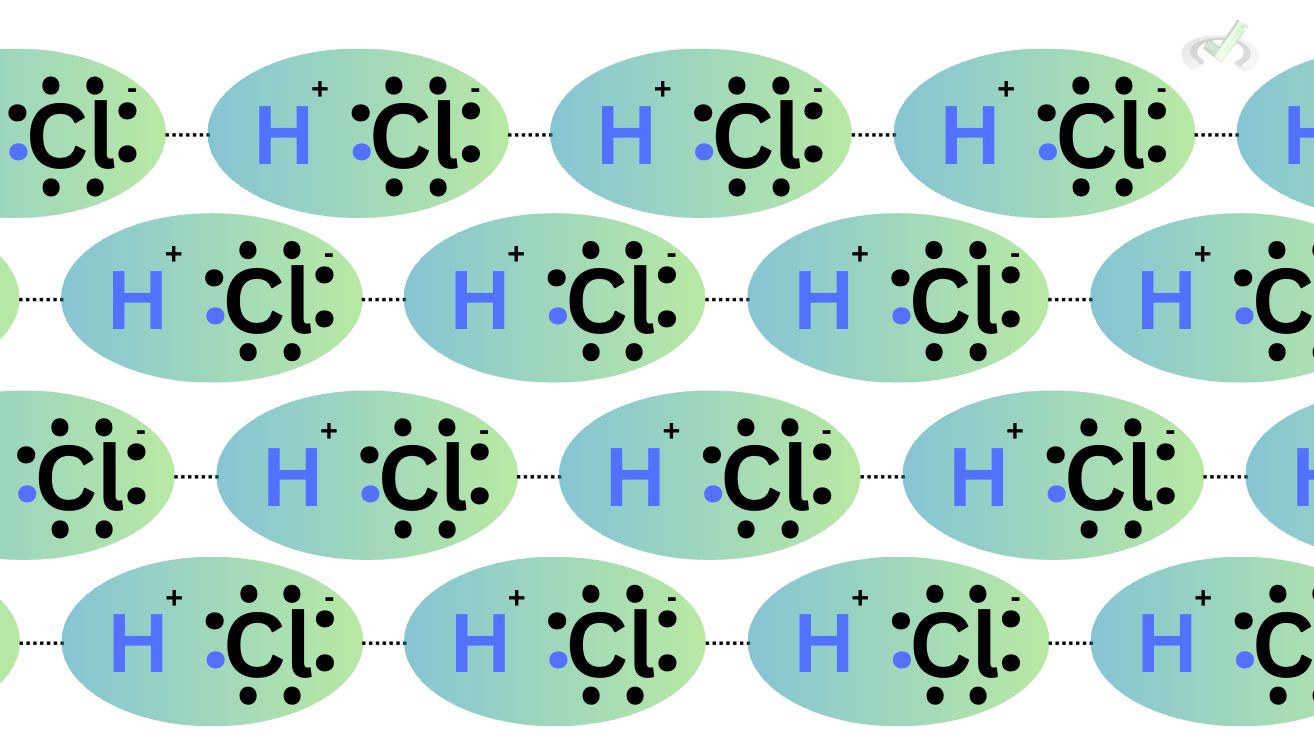
HCl is an example of a polar molecule because it does not equally distribute its electrons to the bonding atoms. The electron distribution is influenced by electronegativity. Electrons tend to move towards the more electronegative side when something is electronegative. For hydrogen chloride, chlorine is more electronegative. It will, therefore, have an electron-dense region, and lead to a partially negative pole on its side. Hydrogen, on the other hand, becomes the partially positive pole. Through the dipole-dipole interactions, the partially positive hydrogen on one side becomes attracted to the neighboring partially positive chlorine atom of another molecule.
III. Hydrogen Bonding
Hydrogen bonds occur when hydrogen atoms bonded to an electronegative atom are attracted to an electronegative atom of another molecule. We usually see this in water molecules. Water has one oxygen atom bonded to two hydrogen atoms. Oxygen is electronegative, meaning it tends to pull electrons toward itself. Here, we see that the hydrogen atom of a water molecule is attracted to the oxygen atom of another water molecule.
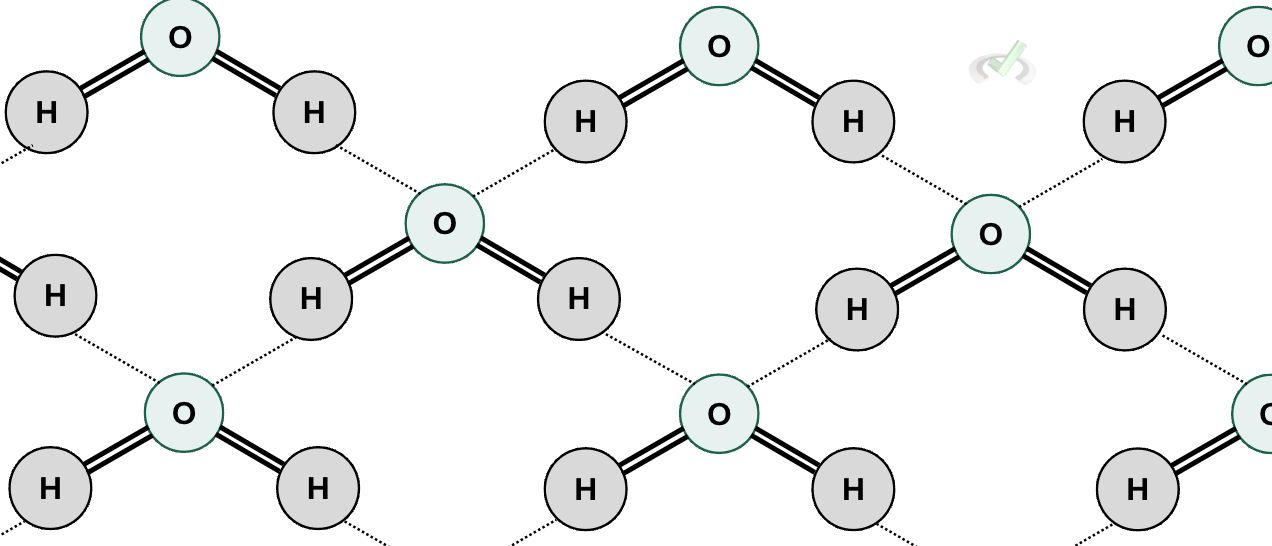
Elements like oxygen, fluorine, or nitrogen are associated with having high electronegativities, which makes them more likely to form strong hydrogen bonds. The presence of hydrogen bonds influences a molecule's physical properties. Molecules of the same size as water typically have low boiling and melting points. Still, the hydrogen bonds between the water molecules allow them to have high boiling and melting points.


Another important effect of hydrogen bonding is cohesion. The tendency of water molecules to stick to each other. We see this in water droplets naturally mixing as one big water blob. Even the tendency of water to allow tiny objects like insects to be on top of the water without breaking is influenced by hydrogen bonds through surface tension.
IV. Conclusion
Intermolecular Forces are often related to bonds since they influence how a molecule interacts with each other. These interactions will usually manifest through physical properties in the said molecule. A van der Waals force is the attraction resulting from a temporary dipole. Here, a molecule's positive end is attracted to another's negative end. Another intermolecular force is dipole-dipole interactions that result from the attraction between dipoles. Lastly, hydrogen bonds form by attracting hydrogen molecules bonded to an electronegative atom and an electronegative atom of another molecule. Van der Waals forces are the weakest, and hydrogen bonds are much stronger than dipole-dipole interactions.
V. Key Terms
- Dipole-dipole interactions - An intermolecular force due to the attraction between permanent dipoles.
- Hydrogen bonds - A stronger type of dipole-dipole interaction wherein hydrogen atoms are attracted to an electronegative atom of another molecule.
- Intermolecular force - The interaction between molecules.
- Van der Waals force - An intermolecular force due to the attraction between temporary dipoles.
VI. Practice Questions
Sample Practice Question 1
Which of the following is the correct arrangement of intermolecular forces from strongest to weakest?
A. Hydrogen bond, Van der Waals force, DIpole-Dipole interactions
B. Van der Waals force, Dipole-Dipole interactions, hydrogen bond
C. Hydrogen bond, Dipole-Dipole interactions, Van der Waals force
D. Van der Waals force, hydrogen bond, Dipole-Dipole Interactions
Ans. C
Sample Practice Question 2
Which of the following is not true?
A. Water molecules are cohesive due to hydrogen bonds.
B. Hydrogen bonds increase water’s surface tension.
C. A dipole is a molecule with a negative pole and a positive pole.
D. Van der Waals forces are present in dipole molecules.
Ans. D







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
