We previously discussed that chemical kinetics is concerned with the rate of a chemical reaction. This rate is basically how fast a reaction occurs. We also learned that some reactions require multiple steps, and these steps lead to the formation of the reaction's final products.
Before we get into the nitty gritty of rate laws and calculate actual values that determine how fast a reaction occurs, let’s first get into the theoretical side of things. In order to understand how chemical kinetics occur, it’s important to explore the theories and factors that affect chemical kinetics.
I. Collision Theory
The first theory we’ll look at is collision theory. This can be thought of as an extension of kinetic molecular theory, which assumes that molecules move around. In collision theory, we examine the collisions between reactants that lead to the formation of products.
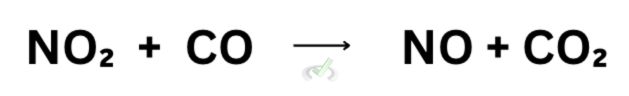
Let’s say we have a jar with gas molecules inside, and let’s use the reaction between nitrogen dioxide (NO₂) and carbon monoxide (CO). The premise of collision theory is that when molecules collide at the perfect angle and with sufficient energy, they react to form products.
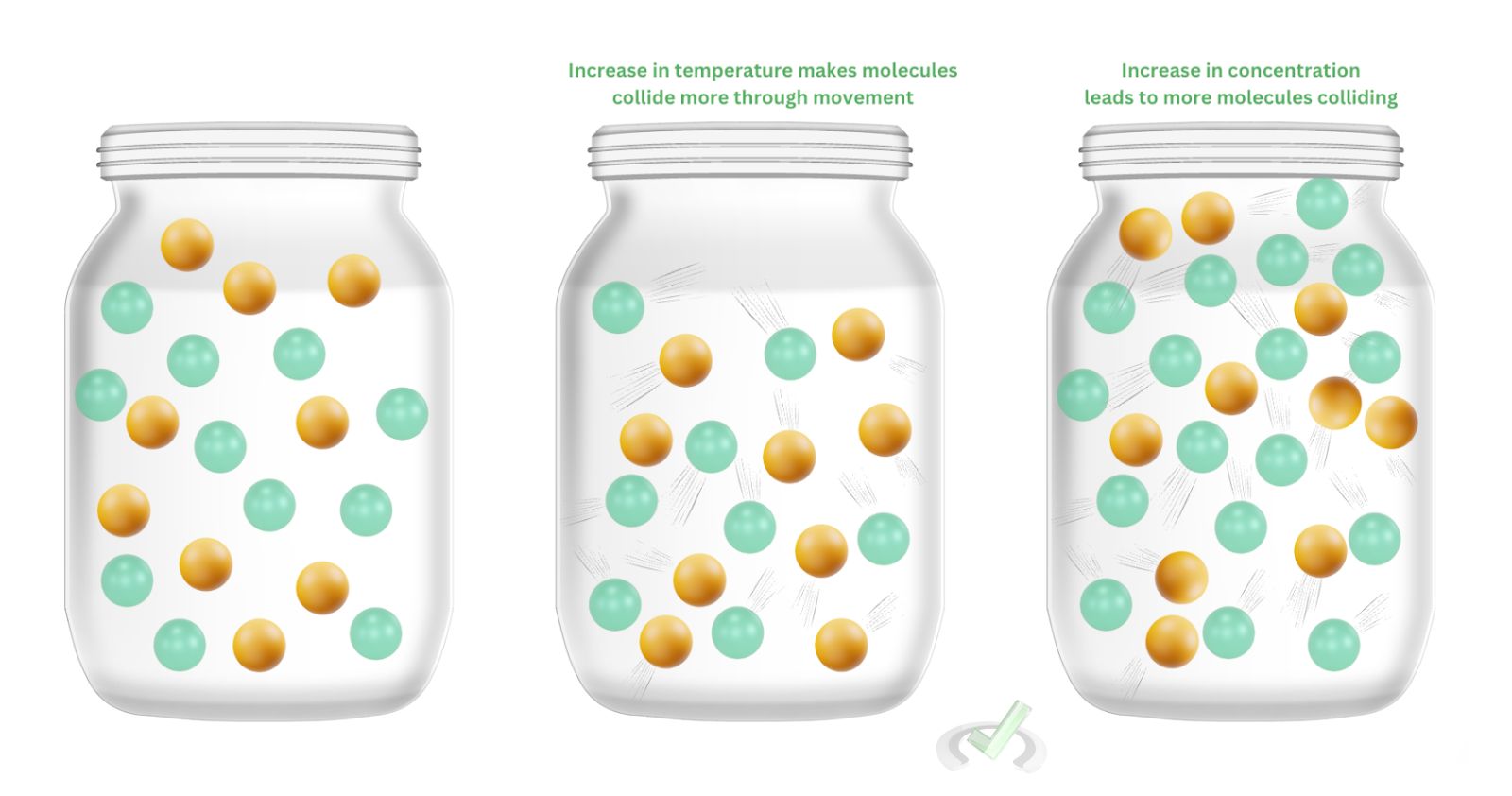
Under the collision theory, there are two factors that can increase the likelihood of molecules colliding with each other. One of them is by increasing the temperature. Increasing an object’s temperature makes molecules move faster. In this theory, by increasing the temperature, the molecules move a lot more, making the reactants bump against each other and react more to make products.
Another factor that increases the likelihood of forming a product in a chemical reaction is increasing the concentration of the reactants. When we put in more molecules of nitrogen dioxide and carbon dioxide, we allow more molecules to collide against each other. When a lot of these molecules collide, more products form.II. Transition State Theory
Now, let’s examine how chemical reactions take place by examining the energy changes throughout the reaction.
The image at the right represents the energy change during a reaction as reactants form into products. Using the chemical reaction between nitrogen dioxide and carbon monoxide, let’s discuss the transition state theory.
At the start of the reaction, the reactants have intermediate energy. Remember that the higher the energy, the less stable a chemical species is. Once the reactants collide and interact, it forms a transition state. The reaction between some nitrogen dioxide molecules leads to the formation of nitrate which acts as an intermediate.
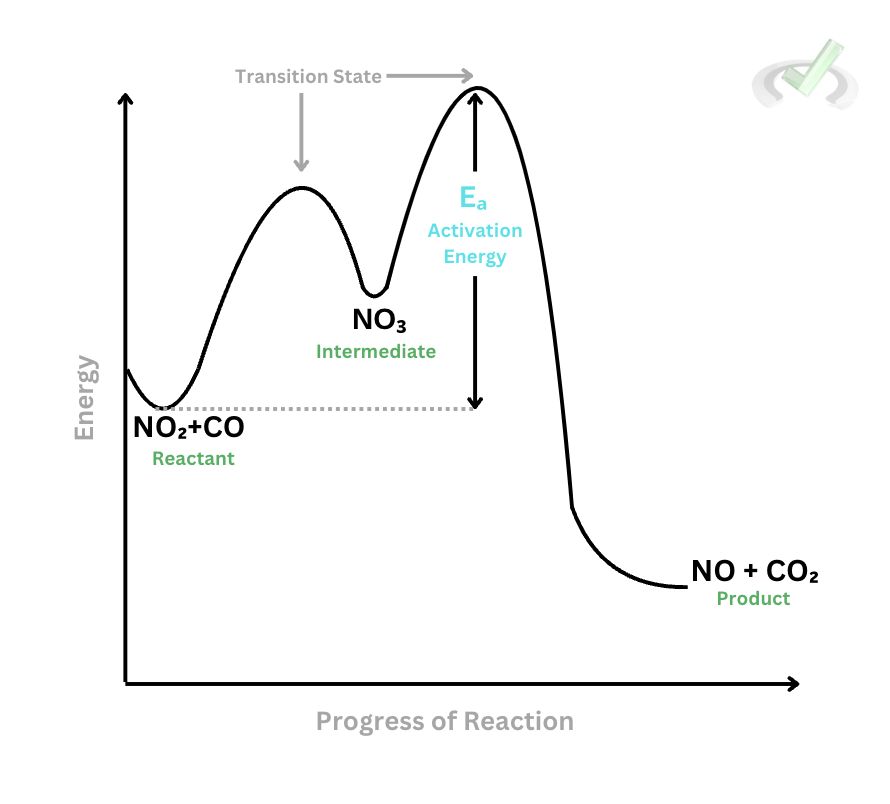
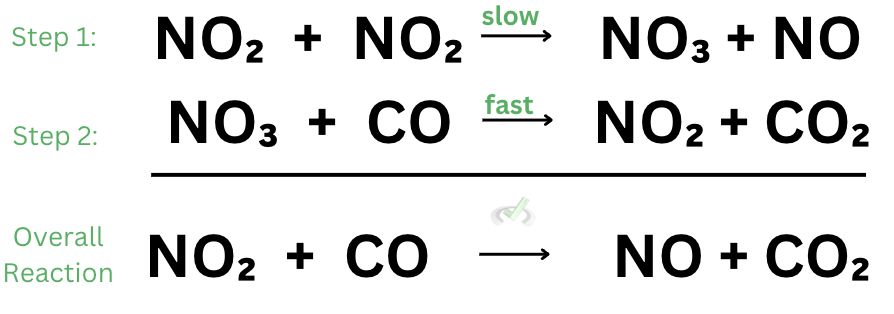
In this step, nitrate and carbon monoxide react with each other. Since nitrate is consumed just after it has been produced, it disappears from the reaction. Notice how when we create the final products, the energy is at its peak. The state at which the energy peaks is known as the transition state, as this is where a lot of energy is used up to make new species.
Once the product forms, the energy lowers which indicates that the products are now more stable than the reactants.
The Activation Energy (Eₐ) is the energy difference between the reactants and the transition state. This is also known as the energy required to break the bonds between the reactants and form bonds in products. According to the transition state theory, a high activation energy results in a slower reaction rate since there will be fewer molecules that can overcome the activation energy barrier.
Think of the activation energy barrier as the energy the reactants need to push through the reaction. Naturally, when a reaction requires tremendous energy to form products, there will be fewer molecules capable of pushing through this barrier and surpassing this energy barrier. It’s sort of similar to a marathon. A lot of people want to get to the finish line, but as the goal gets farther and farther (higher activation energy), more people (molecules) get tired and quit, and only a few actually reach the finish line (transition state).
III. Conclusion
Understanding chemical kinetics relies heavily on two theories: collision and transition state theory. Collision theory states that molecules collide at a perfect orientation to form new products. This theory covers two factors that increase the likelihood of reactants colliding with each other and forming products. One factor is temperature. By increasing the temperature, molecules tend to move much more vigorously, thereby increasing the chances of reactants bumping into each other at the perfect angle, leading to the formation of more products. Another factor is concentration. By increasing the number of reactants, more reactants will collide with each other, making more products. Another theory that explains chemical kinetics is the transition state theory, which basically looks at the energy changes in a chemical reaction from the reactants all the way to the formation of products.
IV. Key Terms
- Activation Energy - Minimum energy needed for a chemical reaction to take place. It is also defined by the energy difference between products and the transition state.
- Collision Theory - A theory that explains the formation of products through effective collisions between reactants.
- Transition State Theory - A framework that describes chemical reactions through the changes in energy from reactants to the formation of products.
V. Practice Questions
Sample Practice Question 1
Which of the following is not accurate?
A. The transition state is a transient state that represents the maximum energy in a chemical reaction.
B. The activation energy determines the rate of reaction.
C. All collisions between molecules will lead to product formation.
D. Reactants must surpass an energy barrier to reach a transition state.
Ans. C
Sample Practice Question 2
An intermediate:
A. Determines the rate of reaction.
B. Functions in the same way as a catalyst.
C. Exceeds the temperature required to form a product.
D. Gets consumed after formation.
Ans. D







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
