It’s without a doubt that batteries are an essential part of our daily lives: from the batteries that power our TV remotes to the ones that power our much needed smartphones, we have to give a shout out to Allesandro Volta and colleagues for giving us this much needed invention!
To understand how this essential tool works in our favor, we must understand the basics of electrochemistry, also piggybacking off of the basics of oxidation-reduction reactions, as we’ll see later in the overview.
After covering this chapter overview, you’ll be equipped with the basic fundamentals of electrochemistry and will have a sound foundation when covering our more in depth articles. Let’s dive in!
Electrochemistry on the MCAT: What You Need to Know
Topics on general chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
Here at MCAT Mastery, we calculated around a 5-7 question average that will cover chemical kinetics. These don’t necessarily have to be asked in the context of general chemistry as these questions can also have applications in organic chemistry and biochemistry!
Introductory general chemistry accounts for 30% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Electrochemistry
As mentioned above, the topics covered in electrochemistry should also be a good refresher for redox reactions as well as some of the topics covered in equilibrium!
It’d be beneficial for you if you can study these topics in conjunction and within the same time frame as you’ll clearly be able to see the overlap and bridges between the materials.
1. Basic Setup of Electrochemical Cell
The main idea for an electrochemical cell is that it utilizes oxidation-reduction reactions in order to generate a current — recall that a current (I) refers to the flow of charges. Within all electrochemical cells, there are 3 main components:
A. Anode Electrode: Oxidation Occurs
B. Cathode Electrode: Reduction Occurs
C. External Circuit Unit: Allows for Current Flow
Below is a typical setup for an electrochemical cell! When we get into the different types of electrochemical cells, we’ll see that there are a couple of key differences between them; however, the above 3 components remain the same.
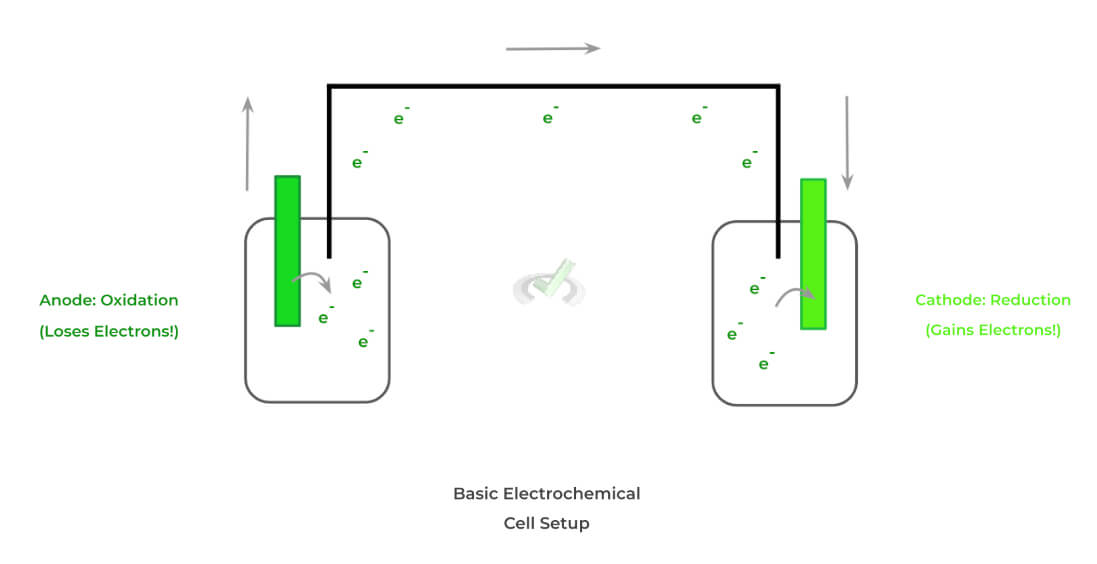
Recall also the basic, opposing definitions of oxidation and reduction: the former refers to losing electrons while the latter refers to the gain of electrons. The tendency of one electrode to lose electrons and the other gains drives the generation of a current!
Full Study Notes : Basic Setup of Electrochemical Cell on the MCAT
For more in-depth content review on the foundations of electrochemical cells, check out these detailed lesson notes created by top MCAT scorers.
2. Redox Chemistry of Electrochemical Cells
As mentioned above, the driving force behind electrochemical cells is the movement of electrons that are given up by the oxidized anode to the cathode, which can gain electrons and subsequently be reduced.
An important value to understand is the standard reduction potential (E˚) which is a value that measures the tendency of a species to be reduced. The more positive E˚ is, the more likely the species is to be reduced. Conversely, the less positive, the less likely it is to be reduced.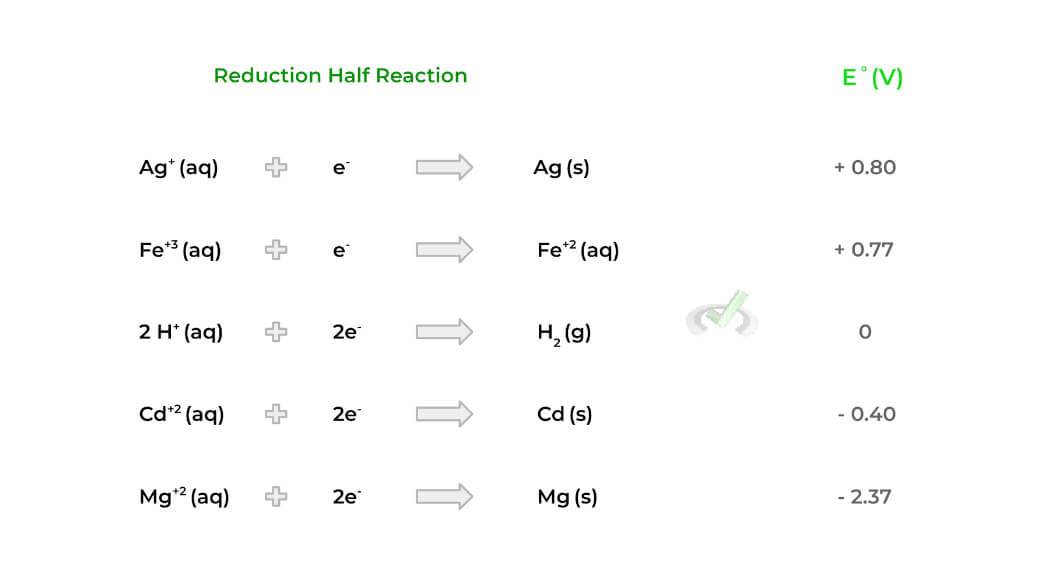
All the standard reduction potential values are in reference to the reduction of a hydrogen ion, which has a value of 0, as shown above. Ideally, we’d want the cathode species to have a more positive E˚compared to the anode!
In fact, another important value to associate with an electrochemical cell is the electromotive force (emf) which is calculated as the difference between the E˚value of the cathode and the E˚ value of the anode!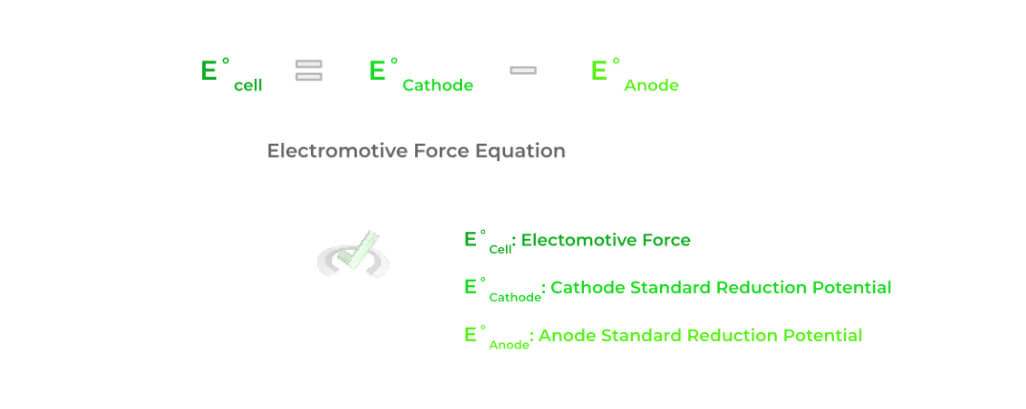
The value of the electromotive force can give a lot of information about the electrochemical cell including its spontaneity, the type of electrochemical cell, and much more, as we’ll cover soon!
Full Study Notes : Redox Chemistry of Electrochemical Cells on the MCAT
For more in-depth content review on how redox chemistry plays a role in electrochemical cells, check out these detailed lesson notes created by top MCAT scorers.
3. Types of Electrochemical Cells
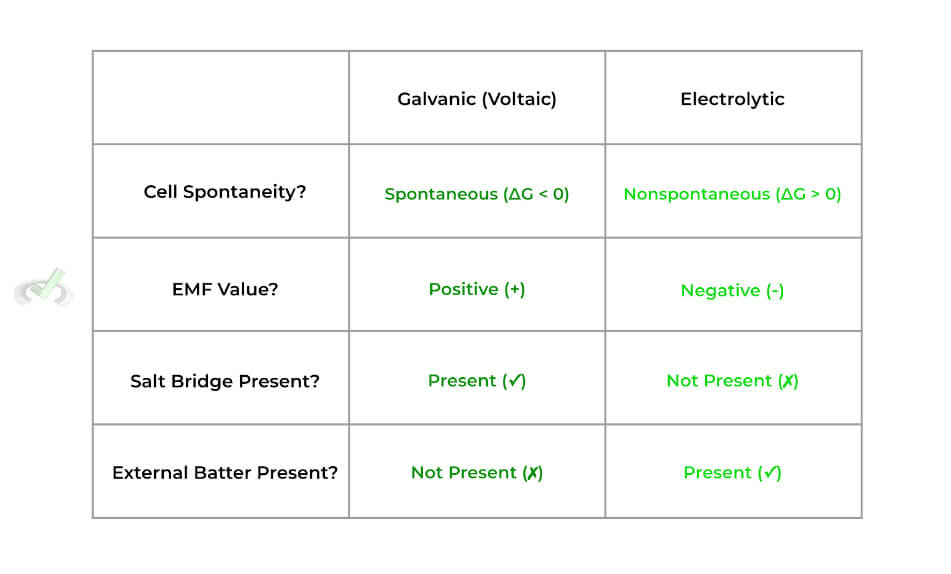
Though there are quite a few different types of electrochemical cells, we’ll primarily focus on 2 main ones as their characteristics and features oppose one another: 1) galvanic (voltaic) cells and 2) electrolytic cells. Shown below is the setup of a galvanic cell!
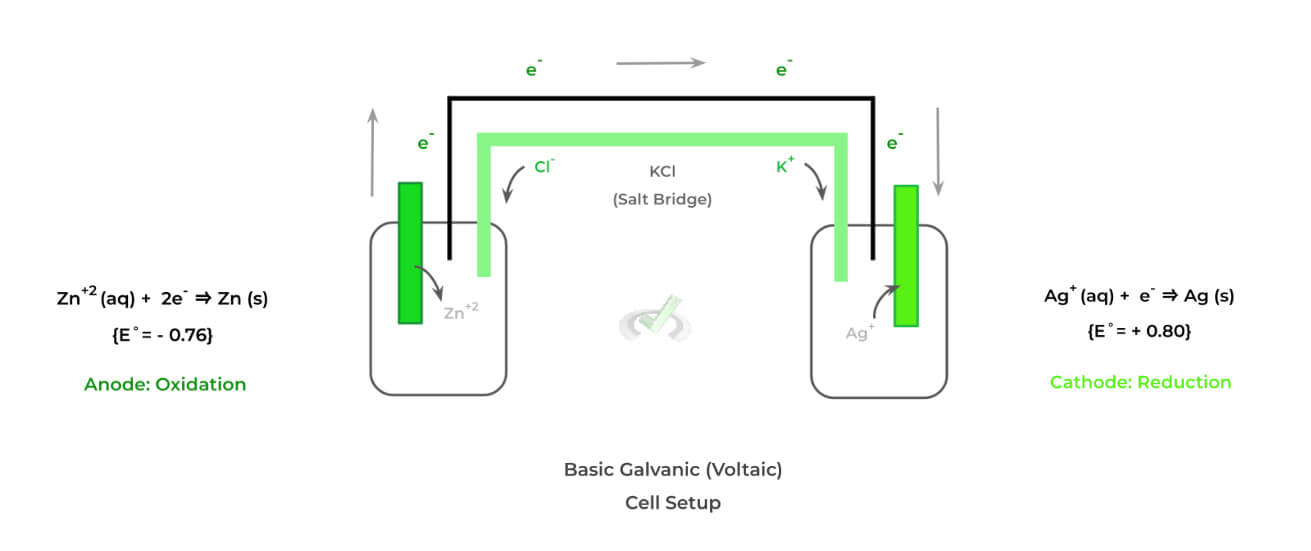
There are a few distinct features that characterize galvanic cells — specifically, these cells are characterized as being spontaneous and having a salt bridge. Let’s look at these characteristics more in depth.
>> Why is it Spontaneous?
The oxidation-reduction reactions are spontaneous because the cathode electrode species (in this case, Ag) has a more positive standard reduction potential and is more likely to be reduced.
As we’ll see in the next section, the change in Gibbs free energy is negative, and the electromotive force of the galvanic cell will be positive.
>> Why is there a Salt Bridge?
The purpose of the salt bridge is to prevent the accumulation of the positive and negative charges on the anode and electrode solutions, respectively.
This prevents the oxidation-reduction reactions from stopping and allows the transfer of electrons and current generation to proceed.
Likewise, shown below is an example of an electrolytic cell which is really characterized as having opposing characteristics and features to galvanic cells.
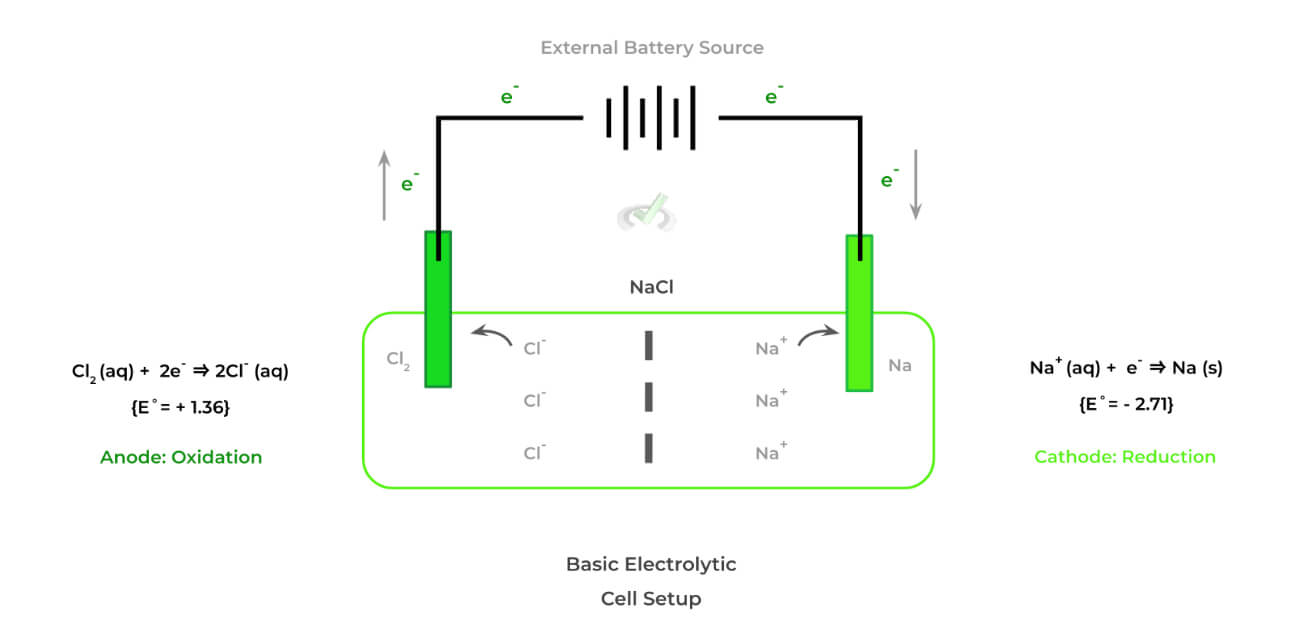
The electrolytic cell also has some features which make it distinct. These cells have opposing characteristics from those of galvanic cells — specifically, they are nonspontaneous and must be powered by an external battery source!
>> Why is it Nonspontaneous?
Look at the E˚values for the anode and cathode: we see that although reduction of Na+ to Na occurs in the cathode, its E˚value is very negative indicating it’s very unlikely to be reduced.
This makes it very unfavorable (and thus nonspontaneous) for the chloride ion to be oxidized at the anode and the sodium ion to be reduced at the
>> Why is there an External Battery Source?
Because the oxidation-reduction reactions in electrolytic cells are unfavorable, an external battery source must be provided in order to power the nonspontaneous reactions.
In a way, you can think of it as a spontaneous reaction (from the galvanic cells of the battery) being coupled to the nonspontaneous redox reactions!
Below is a nice table summation of the differences between the galvanic and electrolytic cells:
Though the redox reactions are nonspontaneous, there are some cases where we do need to use electrolytic cells! Take for example the case above with the electrolysis of sodium chloride.
It’s hard to gain our hands on natural sodium and chloride because they are so reactive in nature! As a result, it’s very beneficial for us to use an electrolytic cell in order to power the nonspontaneous redox reactions which allows us to obtain natural sodium and chloride via their unfavorable reduction and oxidation, respectively!Full Study Notes : Types of Electrochemical Cells on the MCAT
For more in-depth content review on the different types of electrochemical cells, check out these detailed lesson notes created by top MCAT scorers.
4. Equilibrium in Electrochemistry
As you’ve probably noticed if you’ve seen some of our other past chapter overviews, the application of reaction equilibrium will always be a key component in any general chemistry topic from electrochemistry, solutions, and much more!
We already mentioned above that galvanic and electrolytic cells are characterized as being spontaneous and nonspontaneous, respectively. Additionally, we also said that galvanic and electrolytic cells also have positive and negative emf values respectively. The relationship between these 2 values can be given by the equation below: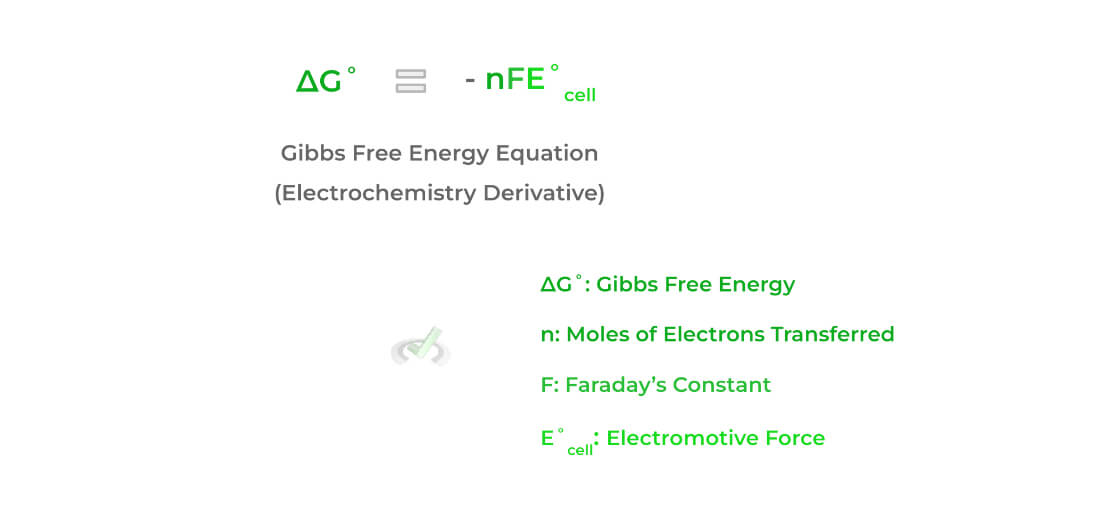
Don’t word too much about the variables of the equation; rather, pay close attention to the negative sign! It now should make sense why the relationship above occurs:
A positive E˚value, as seen in galvanic cells, will still result in a negative Gibbs free energy because multiplying a positive and a negative sign — ultimately resulting in the reactions being spontaneous!
Conversely, a negative E˚value, as seen in electrolytic cells, will still result in a positive Gibbs free energy because multiplying 2 negative signs — ultimately resulting in the reactions being nonspontaneous!Full Study Notes : Equilibrium in Electrochemistry on the MCAT
For more in-depth content review on how equilibrium can be applied to electrochemistry, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about electrochemistry in general chemistry!
Term | Definition |
|---|---|
Electrochemical Cell | A device which utilizes oxidation-reduction reactions in order to generate a current via the flow of electrons |
Anode | The electrode of an electrochemical cell where oxidation takes place |
Cathode | The electrode of an electrochemical cell where reduction takes place |
Standard Reduction Potential | A value which indicates the likelihood that a species will be reduced (i.e. gain electrons) |
Electromotive Force | The difference in the standard reduction potential between the cathode and anode species |
Galvanic (Voltaic) Cell | A type of electrochemical cell which is described as spontaneous and having a positive EMF value |
Salt Bridge | A component of a galvanic (voltaic) cell which prevents the accumulation of charge in the anode and cathode solutions |
Electrolytic Cell | A type of electrochemical cell described as being nonspontaneous and having a negative EMF value; Must be powered by an external battery source |
Additional FAQs - Electrochemistry on the MCAT
What do I Need to Know for Electrochemistry? – MCAT
These topics will then give you the foundation for when you cover and review the types of electrochemical cells as well as understand the application of equilibrium in electrochemistry.
Is the Cathode Positive or Negative? – MCAT
Conversely, a cathode in an electrolytic cell is negatively charged because it’s directly connected to the negative cathode of the external battery source.
What is a Galvanic Cell? – MCAT
What is a Standard Reduction Potential? – MCAT
Additional Reading Links – Study Notes for Electrochemistry on the MCAT
Additional Reading: General Chemistry MCAT Topics:
- Atomic Structure on the MCAT
- Periodic Table on the MCAT
- Bonding and Chemical Reactions on the MCAT
- Chemical Kinetics on the MCAT
- Acids and Bases on the MCAT
- Equilibrium on the MCAT
- Solutions on the MCAT
- Stoichiometry on the MCAT
- The Gas Phase on the MCAT
- Thermochemistry on the MCAT
- Redox Reactions General Chemistry MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
