Electrochemical cells convert chemical energy into electrical energy or vice versa. They are used in many applications, such as batteries and fuel cells. This study note will cover an electrochemical cell's basic setup and functioning.
I. Introduction to Electrochemical Cells
Electrochemical cells can be broadly classified into galvanic (or voltaic) cells and electrolytic cells.
Galvanic Cells
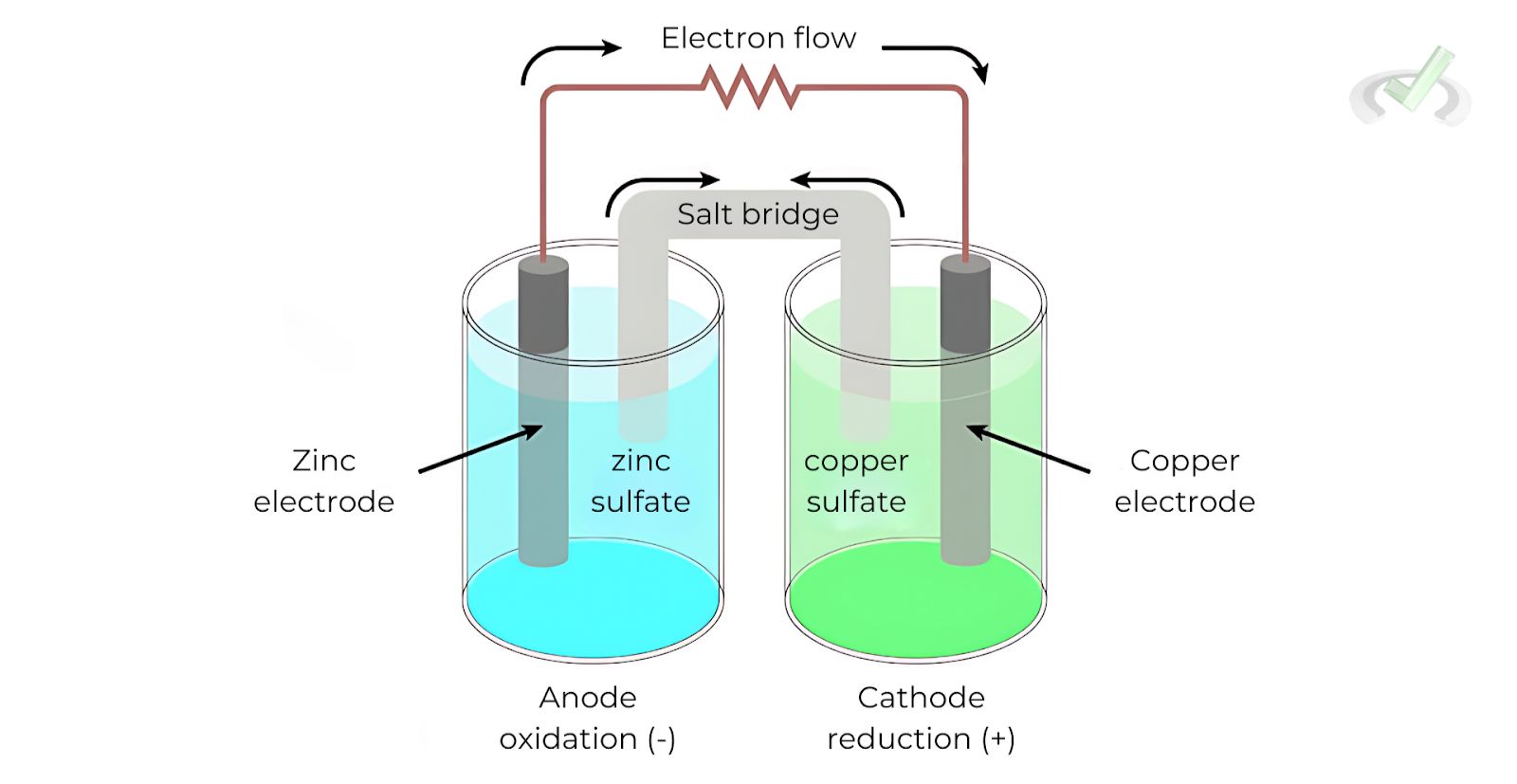
A galvanic cell generates electrical energy from spontaneous chemical reactions. It consists of two different metals connected by a salt bridge or porous membrane, such as a zinc-copper cell.
Electrolytic Cells
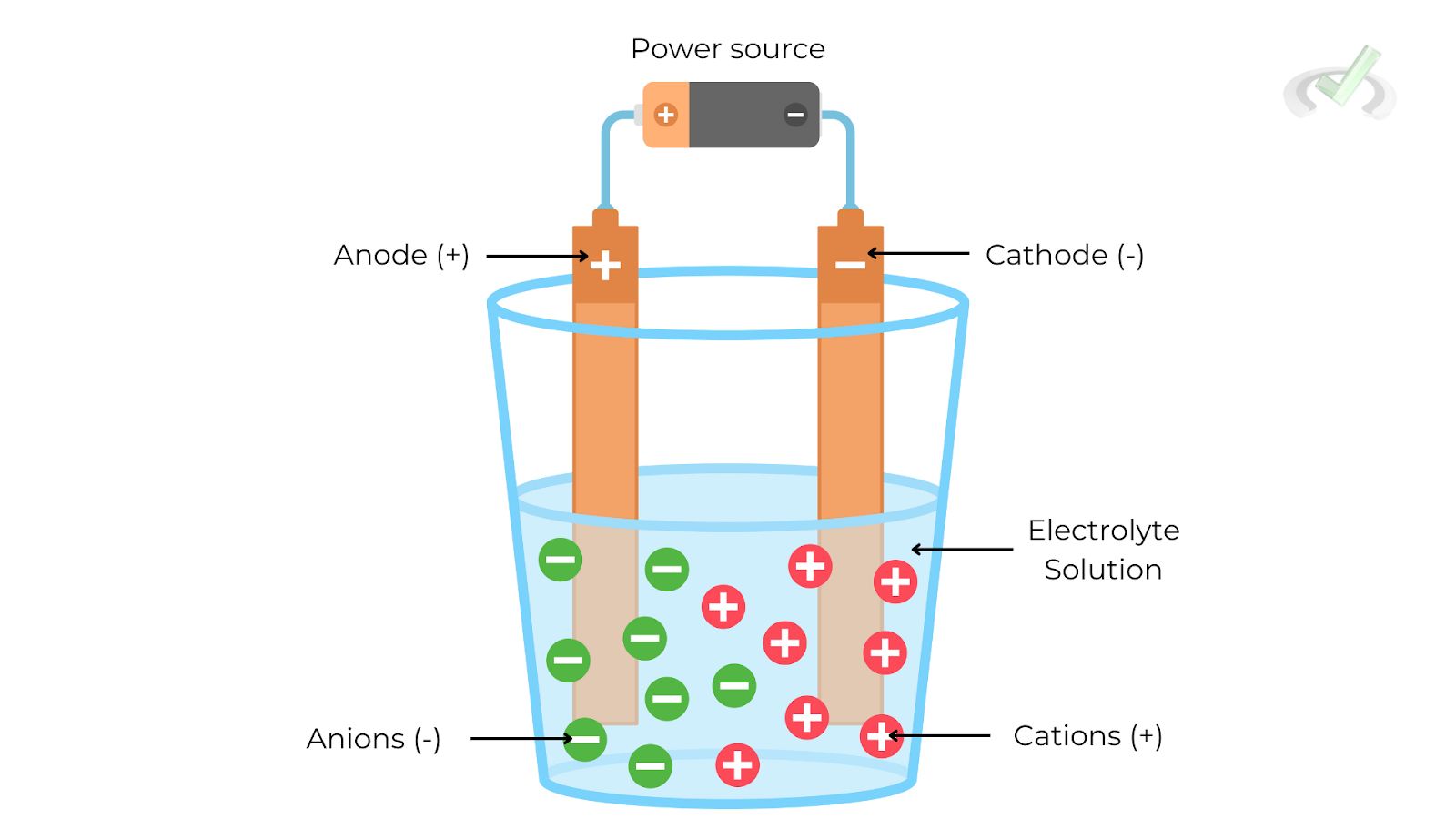
An electrolytic cell uses electrical energy to drive non-spontaneous chemical reactions. These cells are commonly used in processes like electroplating and electrolysis.
II. Components of an Electrochemical Cell
An electrochemical cell has several key components:
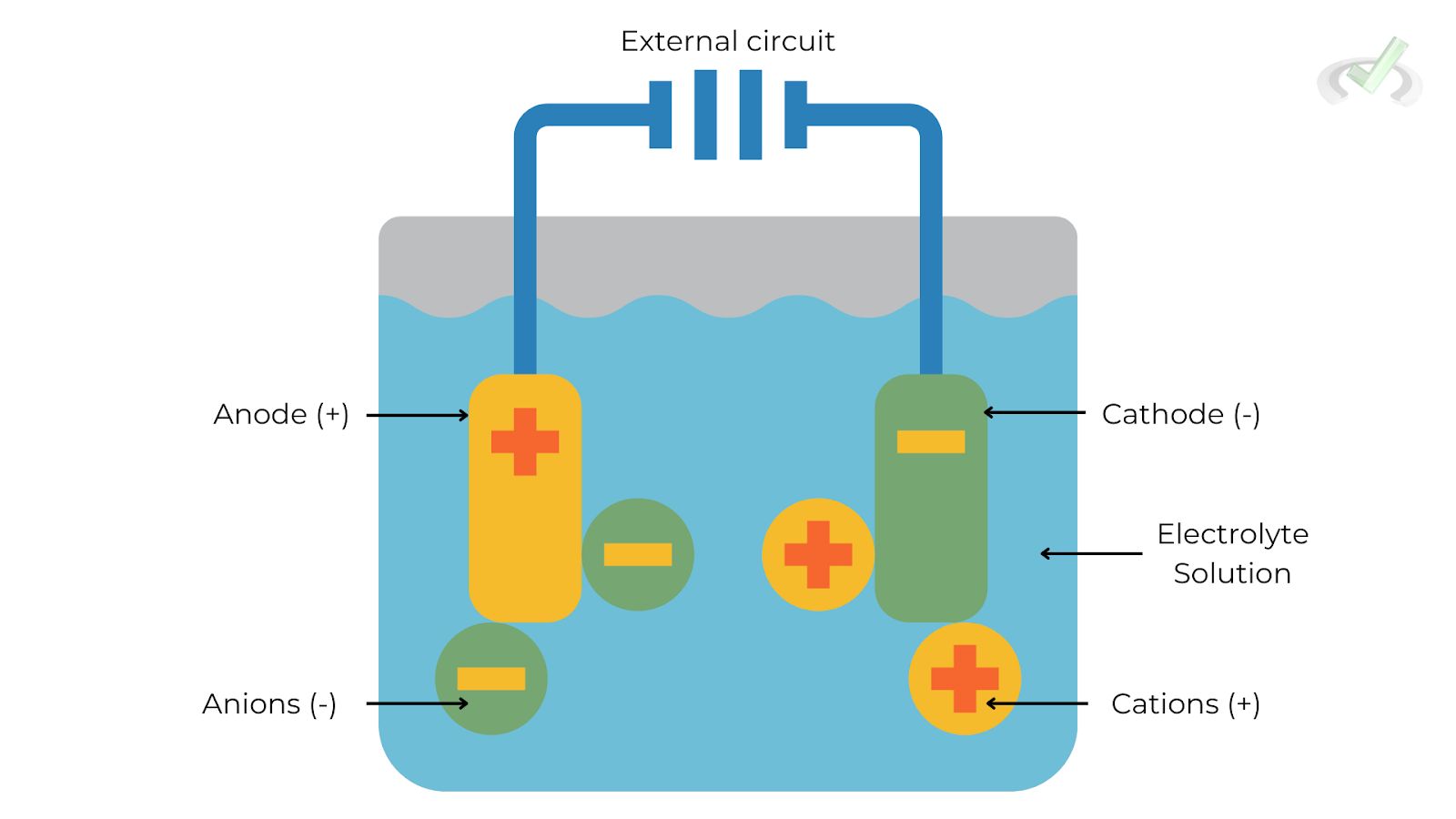
- Anode: The electrode where oxidation (loss of electrons) occurs.
- Cathode: The electrode where reduction (gain of electrons) occurs.
- Electrolyte: A medium that allows the flow of ions but not electrons, facilitating the reaction.
- External Circuit: A path for the flow of electrons from the anode to the cathode.
Example: Zinc-Copper Galvanic Cell
In a zinc-copper galvanic cell:
- Anode (Zinc): Zn(s) → Zn²⁺(aq) + 2e⁻
- Cathode (Copper): Cu²⁺(aq) + 2e⁻ → Cu(s)
III. Working Principle
The basic operation of an electrochemical cell involves the following steps:
- Oxidation at the Anode: The anode undergoes oxidation, releasing electrons into the external circuit.
- Reduction at the Cathode: The cathode undergoes reduction, accepting electrons from the external circuit.
- Ionic Flow: The electrolyte allows ions to flow, maintaining electrical neutrality.
Detailed Explanation
In the zinc-copper cell, zinc loses electrons (oxidation) and becomes Zn²⁺ ions. These electrons travel through the external circuit to the copper electrode, where Cu²⁺ ions gain electrons (reduction) and form solid copper.
Ionic Equation
Overall Reaction: Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
IV. Types of Electrochemical Cells
Galvanic (Voltaic) Cells
These cells convert chemical energy into electrical energy. Common examples include:
- Batteries: Devices that store and provide electrical energy for various applications.
- Fuel Cells: Convert the chemical energy of fuels (like hydrogen) directly into electrical energy.
Electrolytic Cells
These cells use electrical energy to drive chemical reactions. Common examples include:
- Electroplating: Using electricity to coat an object with a layer of metal.
- Electrolysis: Decomposing compounds, like water, into hydrogen and oxygen gases.
V. Applications and Examples
Batteries
Batteries are widely used for portable energy storage. They come in various types, including alkaline, lithium-ion, and lead-acid batteries.
Fuel Cells
Fuel cells are used in vehicles and other applications requiring efficient energy conversion with low emissions.
Electroplating and Electrolysis
Electroplating is used in manufacturing to coat objects with metals like gold or silver. Electrolysis is used to extract metals and split water into hydrogen and oxygen.
VI. Detailed Mechanisms and Measurements
Electron and Ion Flow
The flow of electrons and ions in an electrochemical cell is crucial for its function. The anode releases electrons, which travel through the external circuit to the cathode. Ions in the electrolyte move to balance the charge.
Measuring Cell Potential
The potential difference between the anode and cathode is called the cell potential or voltage. It can be measured using a voltmeter. Cell potential is a measure of the driving force behind electrochemical reactions.
Calculating Gibbs Free Energy
The Gibbs free energy change (ΔG) of a reaction in an electrochemical cell can be calculated using the formula:
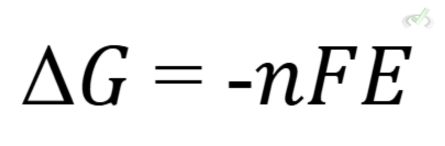
Where n is the number of moles of electrons, F is Faraday's constant, and E is the cell potential.
VII. Bridge/Overlap
Understanding the basic setup of an electrochemical cell is crucial for grasping how redox reactions are applied in various scientific and technological contexts. Here’s how electrochemical cells connect to different fields:
Energy Conversion
Electrochemical cells illustrate how chemical energy can be converted to electrical energy. This is fundamental in energy storage and conversion technologies.
Redox Reactions
The principles of oxidation and reduction are central to the function of electrochemical cells. This connects to broader topics like metabolism in biochemistry and industrial chemical processes.
Material Science
Electrochemical techniques are crucial in developing new materials and improving existing ones, such as creating better battery electrodes or corrosion-resistant coatings.
Thermodynamics
Understanding the energy changes and spontaneity of reactions in electrochemical cells relates to thermodynamic principles. The Gibbs free energy equation ties electrochemical reactions to thermodynamics.
Acid-Base Chemistry
Electrochemical reactions often involve proton (H⁺) transfer, which is linked to acid-base chemistry. For instance, protons move through the electrolyte in a hydrogen fuel cell while electrons travel through the external circuit.
Organic Chemistry
Electrochemical methods are used in organic synthesis. For example, electrochemical oxidation and reduction can create or modify organic molecules.
VIII. Wrap-Up and Key Terms
Electrochemical cells are vital for converting chemical energy to electrical energy. Understanding their setup, components, and functioning helps grasp broader chemistry concepts.
Key Terms
- Anode: The electrode where oxidation occurs.
- Cathode: The electrode where reduction occurs.
- Electrolyte: The medium that allows ionic movement.
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
- Galvanic Cell: Generates electrical energy from chemical reactions.
- Electrolytic Cell: Uses electrical energy to drive chemical reactions.
- Cell Potential: The potential difference between the anode and cathode.
- Gibbs Free Energy: The energy associated with a chemical reaction that can do work.
IX. Practice Questions
Sample Practice Question 1
In a galvanic cell, which electrode undergoes oxidation?
A. Cathode
B. Anode
C. Electrolyte
D. Salt Bridge
Ans. B
Oxidation occurs at the anode, where the material loses electrons.
Sample Practice Question 2
Which of the following is an application of electrolytic cells?
A. Powering a flashlight
B. Electroplating jewelry
C. Running a car engine
D. Lighting a bulb
Ans. B
Electrolytic cells are used in electroplating to coat objects with a tmetal layeretal.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
