Le Chatelier’s Principle helps us understand how a system at equilibrium reacts to changes. It states that if a dynamic equilibrium is disturbed by changing conditions, the equilibrium position moves to counteract the change. This guide will explain the principle, how it applies to different reactions, and connect it to broader chemistry topics.
I. Introduction to Le Chatelier’s Principle
Le Chatelier’s Principle predicts how concentration, temperature, and pressure changes affect a chemical equilibrium. It helps us understand how reactions shift to restore balance.
A. What is Chemical Equilibrium?
Chemical equilibrium happens when the rates of the forward and reverse reactions are equal. At this point, the concentrations of reactants and products stay the same over time. For example, in the reaction:
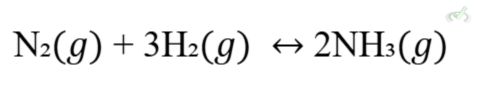
At equilibrium, the rate at which N2 and H2 react to form NH3 equals the rate at which NH3 breaks down into N2 and H2.
II. Factors Affecting Equilibrium
Le Chatelier’s Principle tells us how a system at equilibrium responds to changes. Here are the main factors:
A. Change in Concentration
If the concentration of a reactant or product changes, the equilibrium shifts to oppose that change. This helps the system maintain balance.
Example:
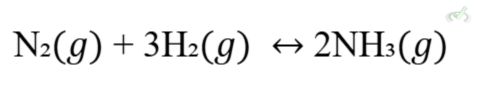
B. Change in Temperature
Temperature changes can also affect equilibrium. The shift direction depends on whether the reaction is exothermic (releases heat) or endothermic (absorbs heat).
Example:
For an exothermic reaction:
A+B↔C+D+heat
For an endothermic reaction:
A+B+heat↔C+D
C. Change in Pressure
Pressure changes only affect reactions involving gases. If the number of gas molecules changes during the reaction, changing the pressure will shift the equilibrium.
Example:
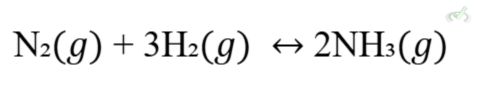
III. Applying Le Chatelier’s Principle to Chemical Reactions
Let's see how Le Chatelier’s Principle applies to various reactions.
A. The Haber Process
The Haber process synthesizes ammonia from nitrogen and hydrogen. It’s an important industrial reaction.
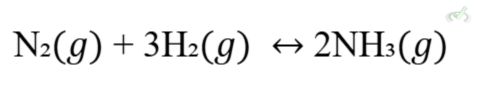
- Increasing concentration: Adding more N2 or H2 shifts the equilibrium to the right, producing more NH3.
- Temperature: Lower temperatures favor the forward reaction (producing ammonia), but too low a temperature slows the reaction rate.
- Pressure: High pressure shifts the equilibrium to the right, favoring ammonia production.
B. The Contact Process
The Contact process produces sulfuric acid, another crucial industrial chemical.
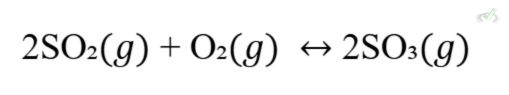
- Increasing concentration: Adding more SO2 or O2 shifts the equilibrium to the right, producing more SO3.
- Temperature: Lower temperatures favor the formation of SO3, but the reaction rate is slower at low temperatures.
- Pressure: High pressure shifts the equilibrium to the right, favoring SO3 production.
IV. Common Types of Reactions Affected by Le Chatelier’s Principle
Different types of reactions respond to changes according to Le Chatelier’s Principle.
A. Reversible Reactions
These reactions can go both forward and backward and are significantly influenced by changes in conditions.
Example:

B. Solubility Equilibria
Solubility equilibria involve the dissolution and precipitation of solids in liquids.
Example:

V. Bridge/Overlap
Understanding Le Chatelier’s Principle helps with other areas of chemistry.
A. Reaction Rates
Knowing how equilibrium shifts can help us understand activation energy and catalysts. Catalysts speed up the rate at which equilibrium is reached without affecting the equilibrium position.
B. Thermodynamics
Le Chatelier’s Principle connects with thermodynamic concepts like enthalpy (heat content) and entropy (disorder). These concepts explain why reactions favor certain conditions and how energy changes drive shifts in equilibrium.
C. Biochemical Systems
In biochemistry, Le Chatelier’s Principle helps explain how metabolic pathways respond to changes. For example, the body maintains homeostasis by shifting biochemical reactions to counteract changes in concentrations of substrates and products.
VI. Wrap-Up and Key Terms
Le Chatelier’s Principle helps us predict how changes in conditions affect chemical equilibrium. It applies to concentration, temperature, and pressure changes.
Key Terms
- Equilibrium: A state where the rates of the forward and reverse reactions are equal.
- Exothermic Reaction: A reaction that releases heat.
- Endothermic Reaction: A reaction that absorbs heat.
- Reversible Reaction: A reaction that can go both forward and backward.
- Solubility Equilibria: The balance between dissolution and precipitation of a solid in a liquid.
VII. Practice Questions
Sample Practice Question 1
How does increasing the concentration of N2 affect the equilibrium in the Haber process?
A. Shifts left
B. No change
C. Shifts right
D. Stops reaction
Ans. C
Increasing the concentration of N2 shifts the equilibrium to the right, producing more NH₃.
Sample Practice Question 2
What happens to the equilibrium of an exothermic reaction when the temperature is increased?
A. Shifts left
B. No change
C. Shifts right
D. Speeds up reaction
Ans. A
Increasing the temperature of an exothermic reaction adds heat, shifting the equilibrium to the left to absorb the extra heat.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
