Chemical equilibrium is the state where the rates of both the forward & reverse reactions are equal. At this point, the reactant and product concentrations remain constant. This guide will help you understand the equilibrium concept in chemical reactions and how it applies to various scenarios.
I. Introduction to Chemical Equilibrium
Chemical equilibrium is an important concept in chemistry. It occurs when a chemical reaction proceeds at the same rate in both directions. This means the concentrations of reactants and products remain unchanged over time, although the reactions continue to occur.
A. Dynamic Equilibrium
In dynamic equilibrium, reactants turn into products, and products turn back into reactants at the same rate. The reactants convert to products, and products convert to reactants continuously, but their overall concentrations remain constant.
Example: In the reaction of nitrogen and hydrogen to form ammonia: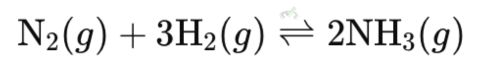
In this reaction, nitrogen and hydrogen combine to form ammonia, while ammonia simultaneously breaks down into nitrogen and hydrogen. At equilibrium, these reactions occur at the same rate, so the nitrogen, hydrogen, and ammonia concentrations remain constant. This balance is achieved with continuous reaction but no net concentration change.
B. The Equilibrium Constant (K) Equilibrium
The equilibrium constant (K) is a number expressing the ratio of product concentrations to reactants at equilibrium.
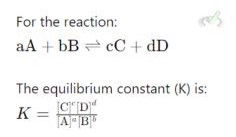
II. Factors Affecting Equilibrium
Several factors can affect the equilibrium position. These include changes in concentration, temperature, and pressure.
A. Le Chatelier’s Principle
Le Chatelier's Principle states that if a system at equilibrium is disturbed, the system will adjust to counteract the disturbance and restore equilibrium.
- Concentration Changes: Adding or removing reactants or products shifts the equilibrium position. The system will shift to counteract the change and re-establish equilibrium. For example, increasing [N2] in the reaction N₂+3H₂⇌2NH₃ shifts the equilibrium towards more NH₃.
- Temperature Changes: Changing the temperature can favor either the forward or reverse reaction. An example is the exothermic reaction, which increases the temperature and shifts the equilibrium to favor the reactants.
- Pressure Changes: Changing the pressure affects reactions involving gases. For example, increasing pressure in N₂+3H₂⇌2NH₃ (fewer gas molecules on the right) shifts the equilibrium toward NH₃.
III. Calculating Equilibrium Concentrations
To calculate equilibrium concentrations, use an ICE table (Initial, Change, Equilibrium).
A. ICE Table Example
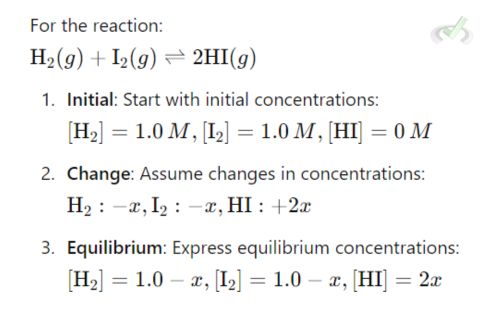
Substitute into the equilibrium expression and solve for x.
IV. Common Equilibrium Problems
Let's look at some typical equilibrium problems and their solutions.
A. Shifts in Equilibrium
Problem: What happens to the equilibrium when more NH₃ is added to N₂+3H₂⇌2NH₃?
Solution: Adding more NH₃ shifts the equilibrium to the left, favoring the formation of N₂ and H₂.B. Calculating K
Problem: For the reaction H₂+I₂⇌2HI, with [H₂]=0.5 M, [I₂]=0.5 M, and [HI]=2.0 M at equilibrium, find K.
Solution:V. Applications of Equilibrium
Chemical equilibrium has many practical applications in industry and everyday life.
A. Industrial Applications
- Haber Process: Produces ammonia for fertilizers. N₂(g)+3H₂(g)⇌2NH₃(g)
- Used in agriculture to produce fertilizers.
- Contact Process: Produces sulfuric acid. 2SO₂(g)+O₂(g)⇌2SO₃(g)
- Used in manufacturing chemicals and fertilizers.
B. Biological Systems
- Oxygen Transport: Hemoglobin binds oxygen in the lungs and releases it in tissues. Hb+O₂⇌HbO₂
- Essential for cellular respiration.
- Essential for cellular respiration.
VI. Bridge/Overlap
Equilibrium concepts extend beyond chemistry and connect with other scientific areas.
A. Thermodynamics
Gibbs Free Energy helps us understand if a reaction will happen on its own. The formula ΔG=ΔH−TΔS shows how energy changes in a reaction. When a reaction is at equilibrium, ΔG=0, meaning the reaction is balanced and no net change happens.
B. Environmental Science
Atmospheric reactions involve equilibrium, such as the formation and breakdown of ozone in our atmosphere. Ozone (O3) is constantly being made and destroyed (O3⇌O2+O), maintaining a balance that protects us from harmful UV rays.
C. Medicine
Drug Reactions use equilibrium principles to understand how drugs interact with the body. For example, enzymes bind with substrates to form enzyme-substrate complexes (E+S⇌ES), which is key to how our bodies process medications.
VII. Wrap-Up and Key Terms
Understanding chemical equilibrium involves mastering several key concepts and terms. Let's review:
Key Terms:
- Dynamic Equilibrium: When the forward and reverse reaction rates are equal.
- Equilibrium Constant (K): Ratio of product concentrations to reactant concentrations at equilibrium.
- Le Chatelier’s Principle: Describes how a system at equilibrium responds to disturbances.
- ICE Table: A method to calculate equilibrium concentrations.
VIII. Practice Questions
Sample Practice Question 1
What happens to the position of equilibrium if the temperature is increased for an exothermic reaction?
A. Shifts to the right
B. Shifts to the left
C. No change
D. Cannot be determined
Ans. B
Increasing the temperature for an exothermic reaction adds heat. According to Le Chatelier’s Principle, the system shifts to favor the endothermic reaction, which in this case is the reverse reaction.
Sample Practice Question 2
Given the reaction N₂(g)+3H₂(g)⇌2NH₃(g), if the concentration of N₂ is increased, what happens to the amount of NH₃?
A. Increases
B. Decreases
C. Stays the same
D. Cannot be determined
Ans. A
Increasing the concentration of N2 shifts the equilibrium to the right. This is according to Le Chatelier’s Principle, which explains the increases in the production of NH₃.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
