Solutions are a fundamental part of chemistry. They are mixtures in which one substance (solute) is dissolved in another (solvent). Understanding the terms and units related to solutions is essential for mastering general chemistry. Let's explore the main concepts and their applications.
I. Introduction to Solutions
A solution is a homogeneous mixture of two or more substances. In a solution, the solute is the substance that gets dissolved, and the solvent is the substance that dissolves.
Example: Saltwater is a solution in which salt (sodium chloride, NaCl) is the solute and water (H₂O) is the solvent.
Importance of Solutions
Solutions are vital in many processes, including biological functions and industrial applications. In biological systems, solutions allow for the transport of essential nutrients and oxygen through the blood, facilitating cellular functions.
Solutions are used in industrial applications, such as chemical manufacturing, where precise concentrations of reactants are necessary for optimal production.
II. Key Terms and Units
Understanding the various terms and units used to describe solutions is crucial in chemistry. Here, we will discuss some key terms and units related to solutions.
Concentration
Concentration describes the amount of solute present in a given quantity of solvent or solution. It is often expressed in various units, which we'll discuss below.
Molarity (M)
Molarity is the number of moles of solute per liter of solution. It is one of the most common ways to express concentration.
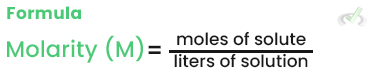
Example: If you dissolve 1 mole of NaCl in 1 liter of water, the solution has a molarity of 1 M.
Molality (m)
Molality is the number of moles of solute per kilogram of solvent. It is used when dealing with temperature changes because it does not change with temperature.
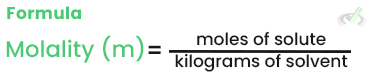
Example: Dissolving 1 mole of NaCl in 1 kilogram of water results in a 1 m solution.
Mass Percent
Mass percent is the solute's mass divided by the solution's total mass, multiplied by 100.
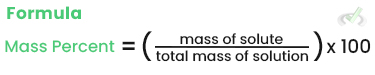
Example: If 10 grams of NaCl is dissolved in 90 grams of water, the mass percent is: (10/100) x 100 = 10%.
Mole Fraction (χ)
The mole fraction is the ratio of the moles of one component to the total moles of all components in the solution.
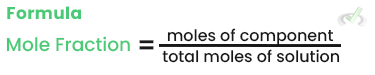
Example: In a solution with 2 moles of NaCl and 8 moles of water, the mole fraction of NaCl is: 2/2+8 = 0.2.
III. Types of Solutions
Solutions can be classified into different types based on the amount of solute present. Let's explore the different types of solutions.
Saturated, Unsaturated, and Supersaturated Solutions
Example: Making rock candy involves creating a supersaturated solution of sugar in water. When the solution cools, excess sugar crystallizes.
IV. Solubility and Factors Affecting It
Solubility refers to the ability of a solute to dissolve in a solvent. Several factors can affect solubility, which we will discuss in this section.
Solubility
- Temperature: Solubility of solids in liquids typically increases with temperature, while gas solubility decreases.
- Pressure: Affects the solubility of gases; higher pressure increases gas solubility (Henry's Law).
- Nature of Solvent and Solute: Polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes (like dissolves like).
V. Calculating Concentrations in Solutions
Calculating the concentration of solutions is an important skill in chemistry. Here, we will go through some example problems to understand how to perform these calculations.
Example Problems
Calculating Molarity:
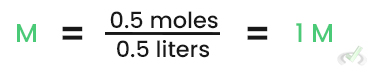
Calculating Molality:

Practical Applications
VI. Illustrations and Equations
Chemical equations help us understand the processes that occur in solutions. Let's look at some common equations and their illustrations.
Chemical Equations in Solution Chemistry
1. Dissolution of Ionic Compounds: When NaCl dissolves in water:
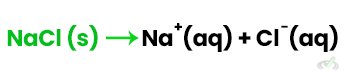
2. Precipitation Reactions: Mixing solutions of silver nitrate (AgNO₃) and sodium chloride (NaCl) forms a precipitate of silver chloride (AgCl):
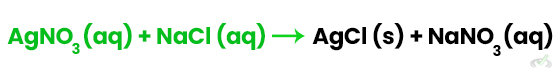
VII. Bridging to Broader Chemistry Concepts
Understanding solutions connects to various broader chemistry concepts. Let's explore some of these connections.
Chemicals Reactions and Equilibrium
Understanding solutions helps in studying chemical reactions and equilibrium. The reactant and product concentrations affects the equilibrium position of many reactions that occur in solution.
Example: In a reaction where equilibrium is established, such as the dissolution of calcium carbonate (CaCO₃) in water, the concentrations of calcium ions (Ca²⁺) and carbonate ions (CO₃²⁻) play a crucial role in determining the solubility product constant (Ksp).
Thermodynamics
Solubility is also connected to thermodynamic principles. The Gibbs free energy change (ΔG) helps predict whether a solute will dissolve spontaneously in a solvent.
Example: The dissolution of ammonium nitrate (NH₄NO₃) in water is endothermic, absorbing heat from the surroundings, which can be analyzed using thermodynamic equations.
Biological Systems
Many biochemical processes depend on solutions. For instance, enzymes in our body function optimally at specific ion concentrations and pH levels, which are influenced by the nature of the solutions they are in.
Example: Blood plasma contains electrolytes like sodium (Na⁺), potassium (K⁺), and calcium (Ca²⁺) ions, which are crucial for maintaining proper cellular function and nerve transmission.
VIII. Wrap-Up and Key Terms
Understanding the main terms and units of solutions is essential for mastering general chemistry concepts. Solutions are involved in numerous processes, from industrial applications to biological systems. By grasping these concepts, you can better understand how different factors influence the behavior and properties of solutions.
Key Terms
IX. Practice Questions
Sample Practice Question 1:
What is the molarity of a solution prepared by dissolving 2 moles of KCl in 1 liter of water?
A. 0.5 M
B. 1 M
C. 2 M
D. 4 M
Ans. C
Molarity (M) is calculated as solute moles divided by solution liters. Here, it is: 2 moles/1 liter = 2 M.
Sample Practice Question 2:
Which of the following factors increases the solubility of gases in liquids?
A. Increasing temperature
B. Decreasing temperature
C. Decreasing pressure
D. Adding a nonpolar solvent
Ans. B
Gas solubility in liquids increases with decreasing temperature.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
