The Periodic Table of Elements looks overwhelming because many terms, colors, and information are dumped on one irregularly shaped rectangular tile. While it contains many numbers, letters, and signs, we'll help break it down so you don't have to feel overwhelmed.
Let's first look at a single element, using carbon as an example. A single tile will always have three main things: the atomic number, atomic mass, and the element name or symbol.
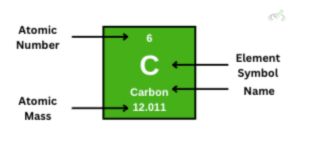
The atomic number is unique to an element. It's what identifies an element; no element can have an atomic number of 6 other than carbon. The atomic number tells us the number of protons present in the atom. It also tells us its order in the periodic table. As you move across a period from left to right, the atomic number increases by one in each tile. The same goes when you move down a group.
The atomic mass will say how many protons and neutrons are in the element. This decimal point is because when looking for the atomic mass, we consider elements of the same kind that can have different neutrons. For now, stick with this: the atomic mass determines the number of protons and neutrons in the atom.
The element symbol and element name are there to identify the element. Most symbols come from the initial letters of the element name (O for oxygen and H for hydrogen). Some elements will have symbols of two letters in their element name (Pt for platinum and Cl for Chlorine). Some elements would have symbols from how they were coined in Latin (Au (Aurum) for Gold and Sn (Stannum) for Tin).
The periodic table of elements has a lot of information that we need for all the elements that make up our entire universe. As we go deeper into the other lessons, you'll soon realize how learning the periodic table might score some points on the MCAT. Let's look at the three main ways we can identify elements.
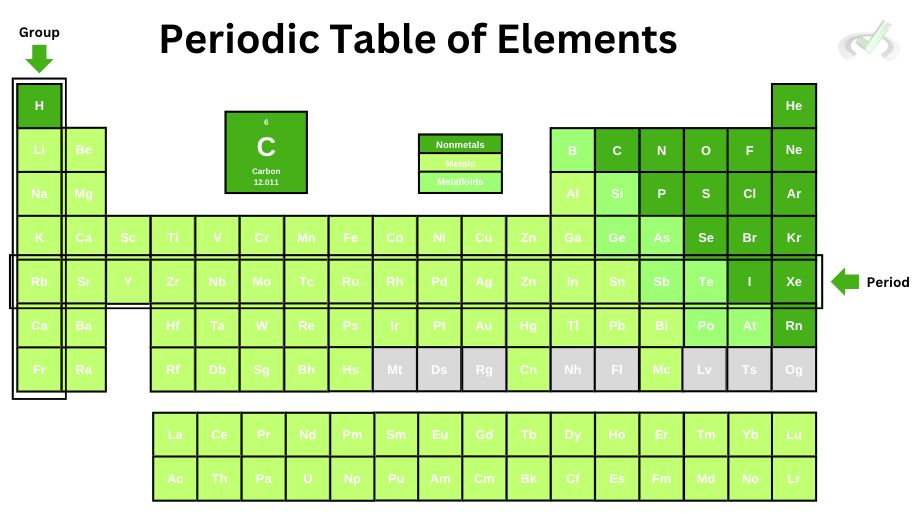
I. Metals
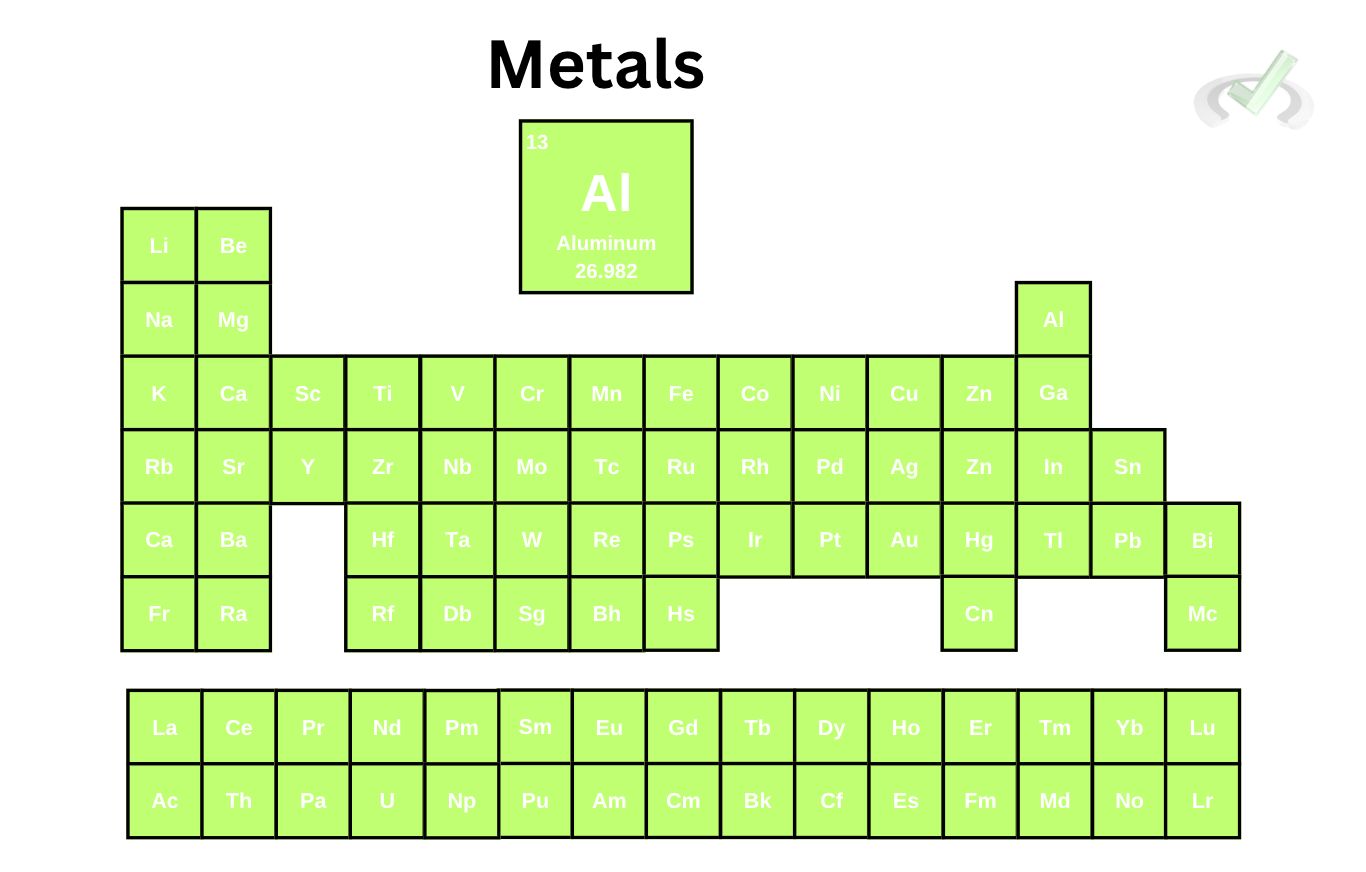
Metallic elements, coming from their name, are very good conductors of electricity. This is why we usually use copper or nickel in electrical wires. They are known for their high electrical conductivity, meaning electrons can move freely across them.
Ionization Energy and Electron Affinity
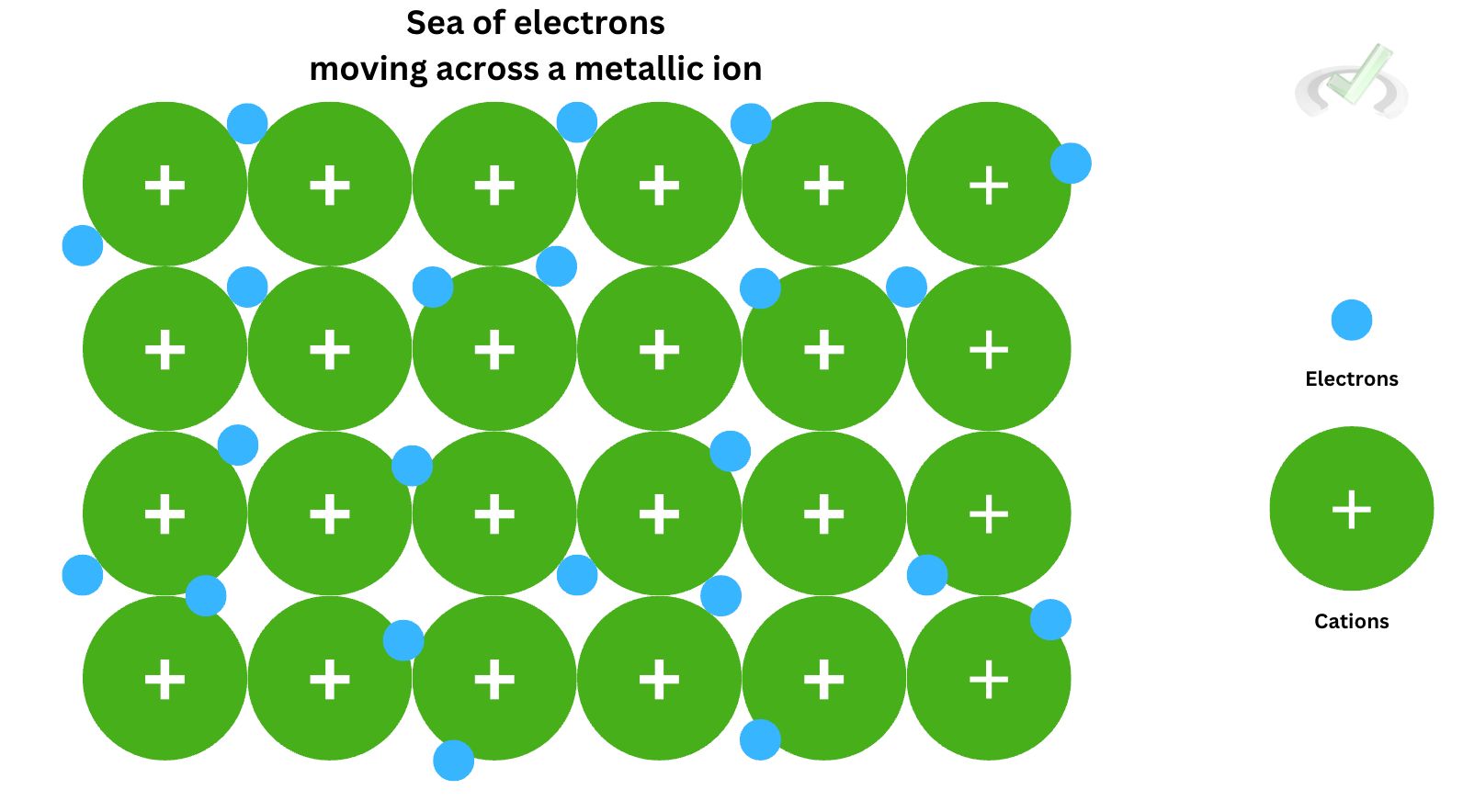
One of the key characteristics of metals is its low ionization energy. This refers to the energy needed to remove an electron from an atom. Since metals have low ionization energy, removing an atom requires little effort. This explains why metals are such good conductors of electricity–electrons can move freely, similar to a sea of electrons because it doesn't require too much to eject an electron.
They have low electron affinity, too. This is the energy emitted or absorbed when an electron is added to an atom. Since this value is low, it doesn't have that much energy to take and keep an electron with it. This also explains why electrons seemingly flow easily in between copper wires. Since electrons don't tend to be kept by metals, they can easily glide their way across a metallic wire.
Metals have low ionization energy and electron affinity. Ionization energy is the energy needed to remove an electron. Because metals have low ionization energy, electrons do not need a lot of energy to remove an electron. It can easily give up an electron, which explains why electrons move freely in metals! Electron affinity is an atom's ability to attract and keep electrons. Similar to this ease in giving up electrons, electron affinity for metals is not very high either since they don't have that much interest in keeping an electron in the first place.Oxidation States
As mentioned in our previous article, metals have multiple oxidation states. Oxidation states show the number of electrons lost or gained to form a chemical bond. This can just be thought of as a charge. This is particularly important in certain chemical reactions. Take Aluminum, for example.

Aluminum is a good reducing agent since it donates electrons in a reduction-oxidation reaction. Reduction-oxidation reactions are important reactions in chemistry that involve donating and gaining electrons across a chemical species to create a reaction. All you have to know is that metals have multiple oxidation states. Since it doesn't have a tight grip on its electrons, it also has multiple oxidation states that make it easy to use in chemical reactions.
II. Nonmetals
Nonmetals are the opposite of metals: they have higher ionization energy and electron affinities. Unlike metals, nonmetals have a more broad character. Depending on the environment, they can be solid, liquid, or gas, and they are very important in biological systems!
Because it has a high ionization energy, it tends to hold on to an electron much stronger. As a result, during chemical bonding, nonmetals will only share or gain electrons. Nonmetals have high electronegativity, meaning they tend to attract chemical species towards themselves. They also have low melting and boiling points. In their solid form, they are not malleable and usually form brittle or soft solids.
A lot of nonmetals are gases at room temperature. Still, nonmetals, such as bromine, are also liquid at room temperature. Contrary to popular belief, carbon can be solid at room temperature. Metals are shiny and highly conductive, whereas nonmetals don't have the same luster. In gaseous form, nonmetals can be colorless or have color. Nonmetals are poor conductors of heat and electricity since they don't have free electrons as opposed to metals.
We see nonmetals in many organic compounds because they are important for biological systems. The four major elements of life (Carbon, Hydrogen, Oxygen, and Nitrogen) are all nonmetals important in the natural world. Even our DNA is made of a lot of nonmetals!
III. Metalloids
Metalloids are elements that have characteristics similar to both metals and nonmetals. Their characteristics depend on the chemical species they're interacting with or their environment.They are also semiconductors, which is why silicon and germanium are usually used in semiconductors. This means they can conduct and insulate electricity. These elements are considered a mix of metals and nonmetals because they have intermediate ionization energy and varied electrical and heat conductivity.
IV. Conclusion
The periodic table of elements gives us much information about the elements. Through this, we can also understand chemical behavior and how elements interact. One way of categorizing the elements is by categorizing them as metals, nonmetals, or metalloids. The differences in their properties can be seen in the table below.
Characteristics | Metals | Metalloids | Nonmetals |
|---|---|---|---|
Physical | Shiny and hard | Dull | Dull, soft, and brittle |
Ionization Energy | Low | Moderate | High |
Electronegativity | Low | Moderate | High |
Density | High | Lower than metals | Low |
Electrical and heat conductivity | Good | Moderate | Poor |
Melting point and Boiling point | High | Moderate | Low |
V. Key Terms
- Conductivity - A measure of how easily electrons (electrical conductivity) or heat (heat conductivity) can move through a substance.
- Density - The mass per one unit of volume of a substance.
- Electronegativity - The tendency of an atom to attract chemical species towards itself.
- Ionization Energy - Ability of an electron to hold on to its electrons.
- Metals - Elements that are shiny, hard, and have high melting and boiling points. They are also characterized by the presence of free electrons.
- Nonmetals - Elements that can be thought of as opposites of metals. They are dull and brittle and have low conductivity. They are characterized by their varied states of matter.
- Metalloids - Elements that have properties similar to metals and nonmetals combined.
VI. Practice Questions
Sample Practice Question 1
Which of the following exhibits both metallic and nonmetallic properties?
A. Te
B. Al
C. Sn
D. Hg
Ans. A
Sample Practice Question 2
All of the following statements are true except:
A. The atomic number indicates the number of protons in an atom.
B. The atomic number increases as you move across the periodic table.
C. Metals have many oxidation states.
D. Nonmetals are good conductors of electricity.
Ans. D







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
