Previously, we learned about the periodic table and how we categorize elements by their metallic or nonmetallic behavior. In this article, we’ll dig a little deeper into the periodic table and tackle one of the key concepts that influence the properties of elements. We’ll also look at how the periodic table can show this behavior as we look at the arrangement of elements. Knowing how we can understand the periodic table of elements is a cheat sheet in chemistry. Once we’re aware of elemental behavior, chemical bonding, reactivity, and other properties could be understood more clearly.
I. Valence Electrons
Valence electrons are electrons in the outermost shell of an atom. These electrons are crucial since they actively participate in chemical bonding. These are the ones involved in creating a bond with another chemical species.
Let’s take carbon as an example.
Carbon has six electrons. Each energy level can take 2n² electrons at most.
For the first energy level, there will be a maximum of 2(1)² = 2 electrons, and for the second energy level, there could be a maximum of 2(2)² = 8 electrons.
Since two electrons can fill out the first energy level, the remaining four of the six electrons of carbon will move onto the second energy level.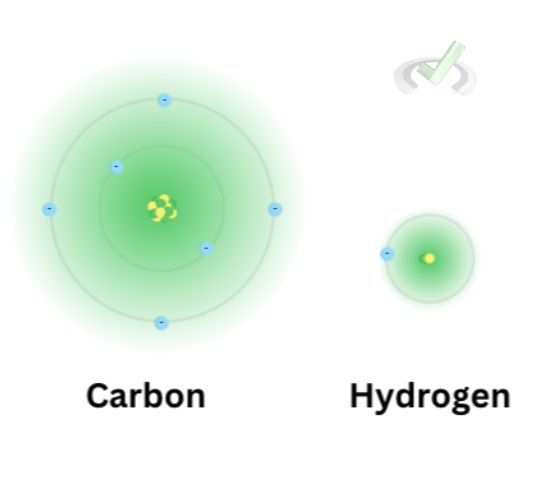
These four electrons at the outer energy level are the valence electrons. These are electrons that will actively participate in chemical bonding.
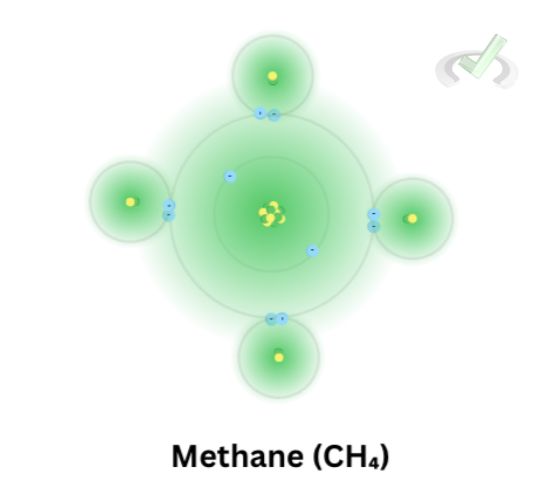
Hydrogen has 1 electron, meaning that it has only 1 valence electron. When carbon and hydrogen bonds, they form a covalent bond by sharing their electrons. Here’s an example of a carbon-hydrogen bond. This bond involves sharing electrons. This way, four hydrogen atoms share a bond with the four valence electrons of carbon
II. Effective Nuclear Charge
We defined the effective nuclear charge as the inward or pulling force of the positive nuclear charge on the valence electrons. Each electron experiences different strength from this inward force depending on the shell it’s in. This positive charge is experienced by an electron on the shell it occupies.

We used the formula Zeff=Z-S. where Zeff is the effective nuclear charge, Z is the number of protons, and S is the number of inner core electrons.
The electrons in the first energy level will experience the greatest pulling force or nuclear charge because it’s the electrons nearest to the nucleus. As we get farther from the nucleus, the inner core electrons will shield some of the positive charge--this makes the value for Zeff decrease as we move up the principal quantum number.
Again, let’s use carbon as an example. The carbon atom has an atomic number of 6, meaning it has six protons and six electrons.
The effective nuclear charge is the number of protons minus the number of inner core electrons. If we take the effective nuclear charge at n = 2, we get:
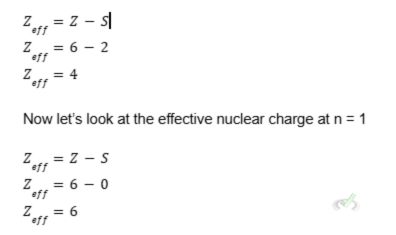
The Zeff for the first shell is larger than n = 2. Note that the number of inner core electrons is zero because there are no electrons between the nucleus and the electrons in the innermost shell. This implies there are no electrons shielding the positive charge from the nucleus. The inner core electrons take the positive attraction from the nucleus through electron shielding.
Let’s try a few more examples.
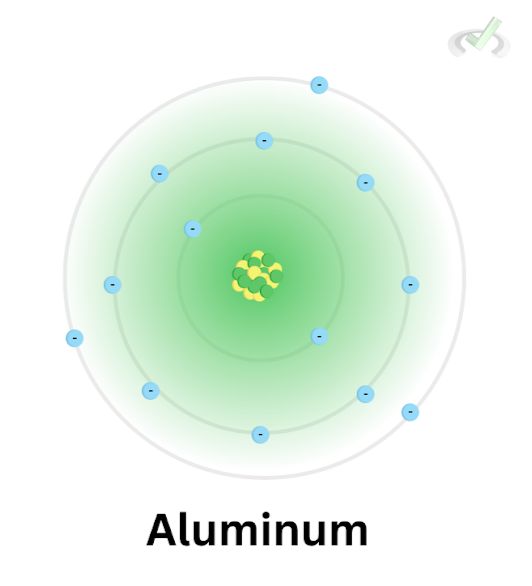
Zeff of Aluminum at n = 3
- Chlorine has an atomic number of 17.
- The total number of inner core electrons is ten since the first ten electrons will fill up the first and second energy levels. The inner core electrons are equal to the total number of electrons between the nucleus and the outer shell.
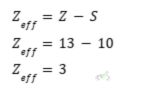
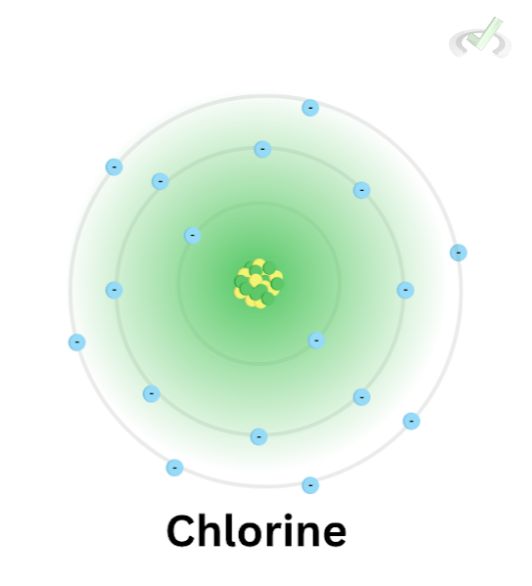
Zeff of Chlorine at n = 3
- Chlorine has an atomic number of 17.
- The total number of inner core electrons is ten since the first ten electrons will fill up the first and second energy levels. The inner core electrons are equal to the total number of electrons between the nucleus and the outer shell.
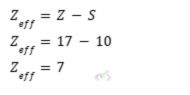
Zeff of Neon at n = 2
- Neon has an atomic number of 10.
- Atoms only fill up until the second energy level. Therefore, all of the inner core electrons are at the second energy level, where the number of inner core electrons is two.
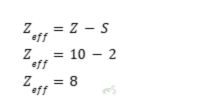
III. The Periodic Table and Valence Electrons
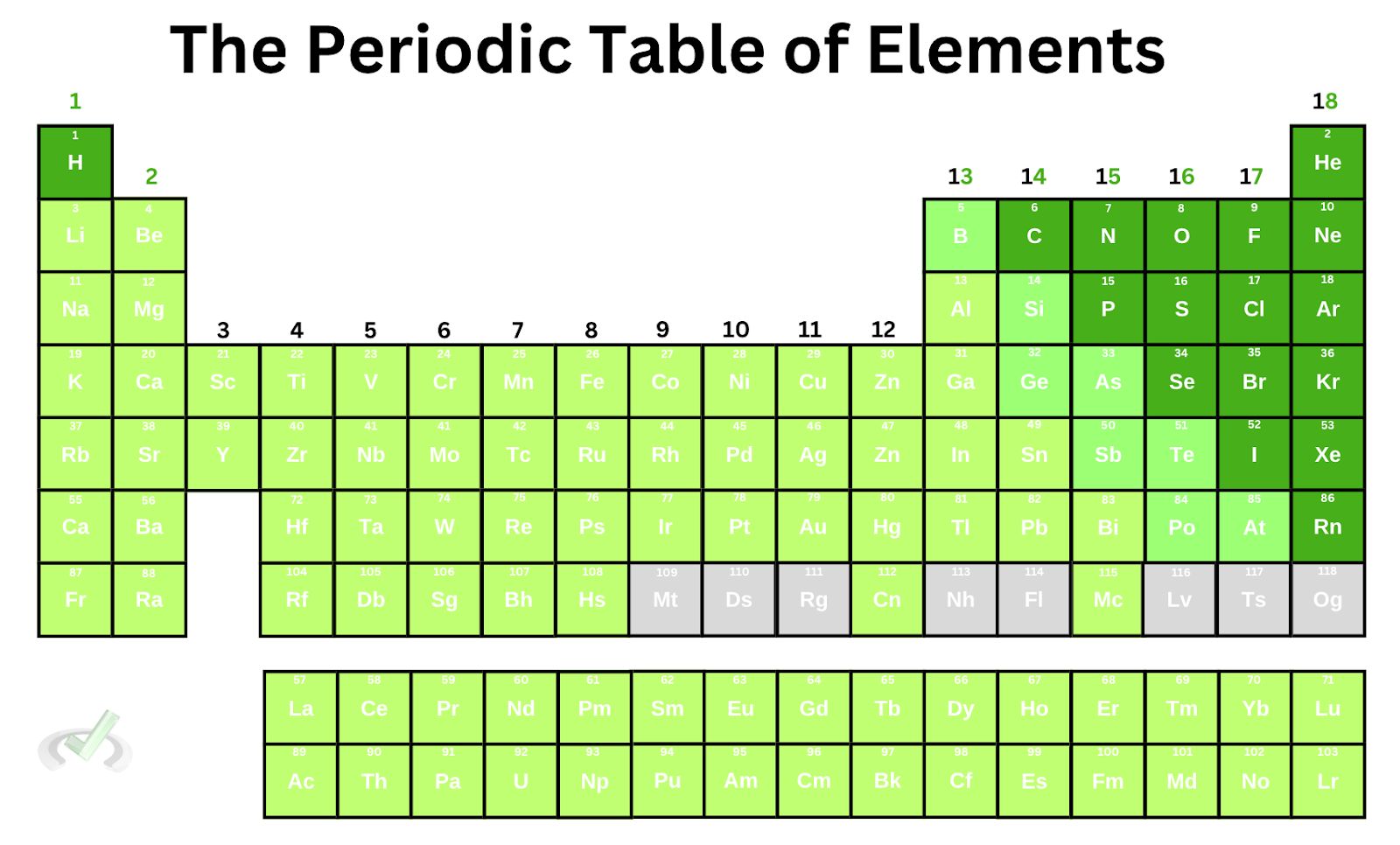
Now that we have a good foundation of valence electrons and effective nuclear charge, let’s look at the periodic table trend on effective nuclear charge.
- The effective nuclear charge increases as we move across a period from left to right.
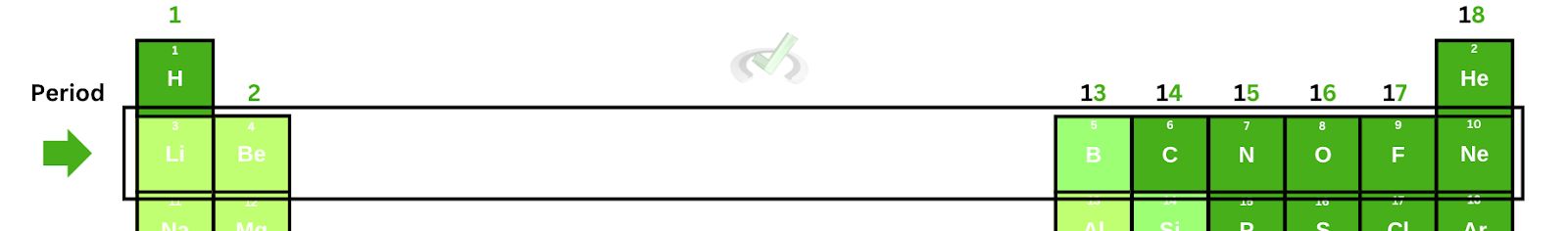
Notice how the elements are arranged in increasing order horizontally. The atomic number increases by 1 as we move from one element to another. Since the atomic number tells us the number of protons and electrons on a neutral atom, this means that as we move across a row, the number of electrons increases, thereby causing an increase in the number of valence electrons.
As the valence electrons increase, the number of inner core electrons remains the same. This happens all the while the number of protons increases as well. Note that the valence electrons are the ones on the outer shell. As we move across a row, we’re adding the electrons on the outermost shell.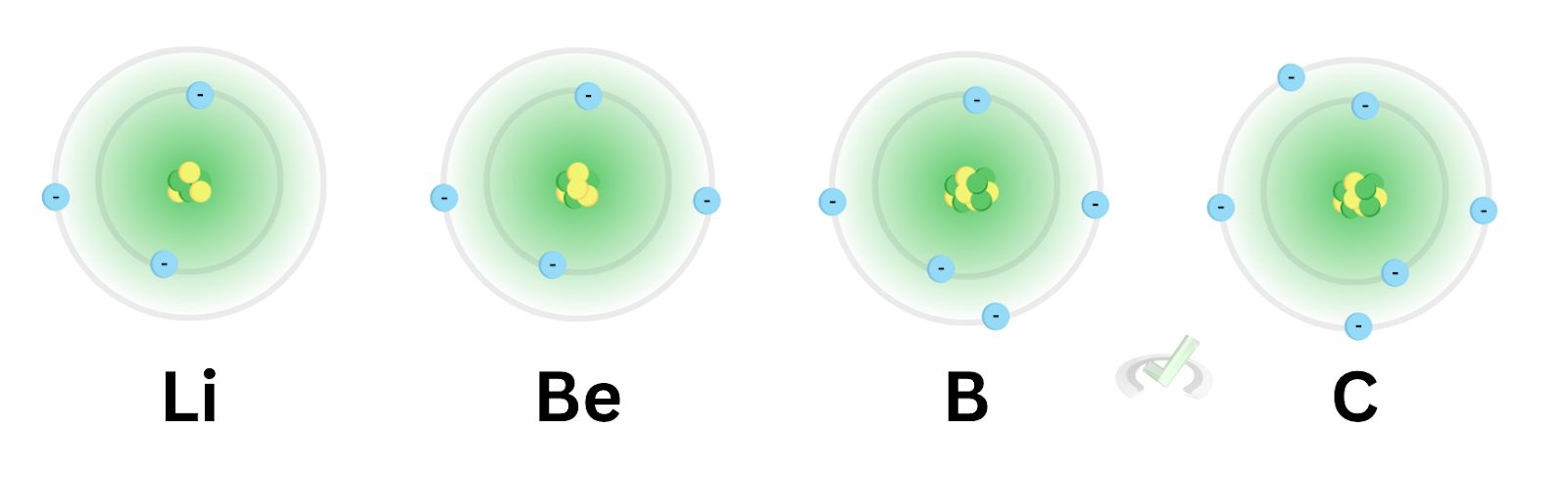
So in lithium, the effective nuclear charge is equal to one. As we move across beryllium, boron, and carbon, the effective nuclear charges increase, with their values being two, three, and four, respectively.
- As we move down a group, the effective nuclear charge remains the same.
When we move down a group, the principal quantum number increases by 1. We also increase the number of electrons and protons. However, the valence electrons down a group are constant. Notice how boron, aluminum, and gallium all have three valence electrons.
As the protons and electrons increase down a group, the value for the effective nuclear charge remains the same since the number of electrons we added to the atom cancels out the number of protons we eventually subtracted from.
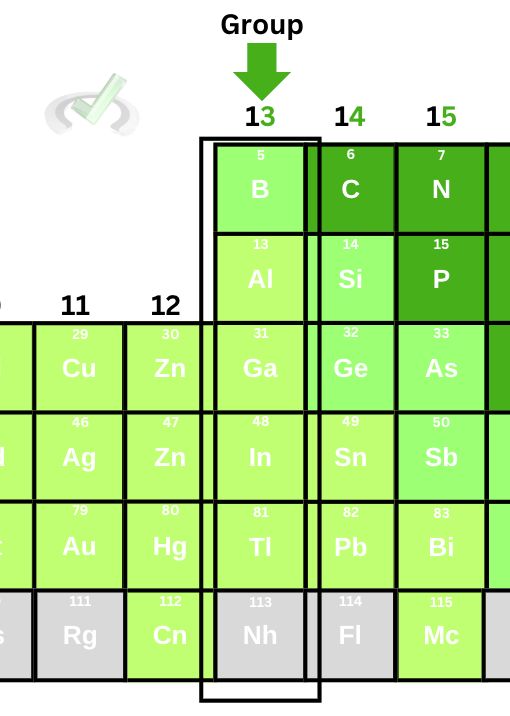

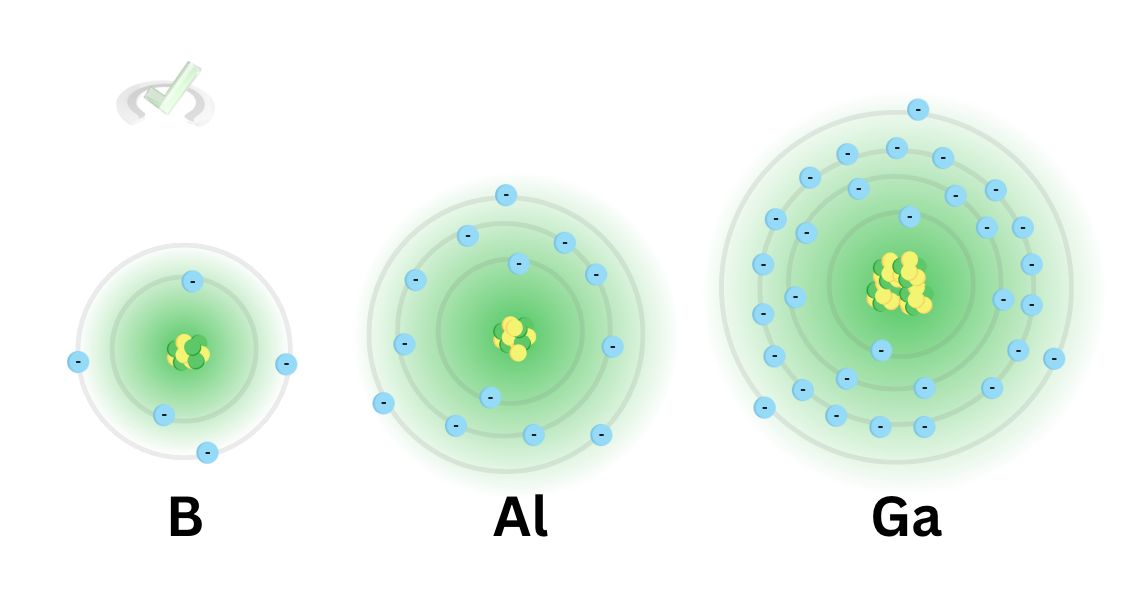
We add a totally filled energy level as we move down a group. This means that nothing changes with the outer core electrons. Whatever we add to the protons and electrons eventually cancels out, making the Zeff relatively constant.
IV. Conclusion
We can notice a few key aspects of effective nuclear charge. As we move across a period, the valence electrons increase, and Zeff increases because we’re increasing the number of protons while keeping the number of inner core electrons constant. As we move down a group, the valence electrons remain the same. Zeff remains constant because we’re adding more inner core electrons by adding another energy level. We also increase the atomic size as the principal quantum number increases.
As we move on to other articles, we’ll notice how Zeff influences the behavior of an element. When Zeff is high, electrons are more attracted to the nucleus. This means it’s difficult to overcome the attraction and remove an electron. This results in a high ionization energy. Electronegativity and reactivity are also properties of elements highly influenced by the effective nuclear charge.
V. Key Terms
- Effective Nuclear Charge (Zeff) - A value of how much attracted an electron is to the nucleus.
- Zeff = Protons - Inner Core Electrons
- Electron Shielding - The effect on the nuclear attraction experienced by outer electrons due to the strong attraction of the inner core electrons with the atomic nucleus.
- Principal Quantum Number (n) = Shows the energy level of an electron.
- Valence Electrons - Electrons on the outermost shell of an electron
VI. Practice Questions
Sample Practice Question 1
The electrons in an atom experience attractive and repulsive forces, what are these forces?
A. Electromagnetic attraction with the positive charges in the surroundings. Electromagnetic repulsion with the negative charges in the surroundings
B. Electrostatic attraction with the protons. Electrostatic repulsion with the electrons.
C. Electromagnetic attraction with the negative charges in the surroundings. Electromagnetic repulsion with the positive charges in the surroundings.
D. Electrostatic attraction with the electrons. Electrostatic repulsion with the protons.
Ans. B
Sample Practice Question 2
Which of the following is NOT TRUE?
A. Atomic size increases as we move down a group.
B. Effective nuclear charge increases as we move across a period.
C. Atomic radius increases as we move down a group.
D. Effective nuclear charge decreases as we move down a group.
Ans. D







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
