The periodic table of elements contains a lot of information in one place. While this collection of letters and numbers may seem daunting, there is a way to get a general picture of all of the elements without going too deep with the numbers.
We discussed effective nuclear charge and briefly discussed valence electrons. We learned that the effective nuclear charge describes the force felt by the electrons in the outermost shell. We also figured that because electrons are between the nucleus and the outermost shell, the inner core electrons lessen the pull felt by the outermost electrons through electron shielding.
We also learned that as we move down a group in the periodic table, the energy level increases by 1, and the effective nuclear charge remains constant. As we move across the periodic table, the number of valence electrons increases as the effective nuclear charge increases.
Now that we've covered how effective nuclear charge affects the attraction between the electrons and the positive nucleus let's move on to how we can use the same concept to understand the periodic table of elements.
I. An Overview of the Periodic Table
Now, if you have a periodic table of elements, you can look at it. If you don't, you can refer to the periodic table of elements below.
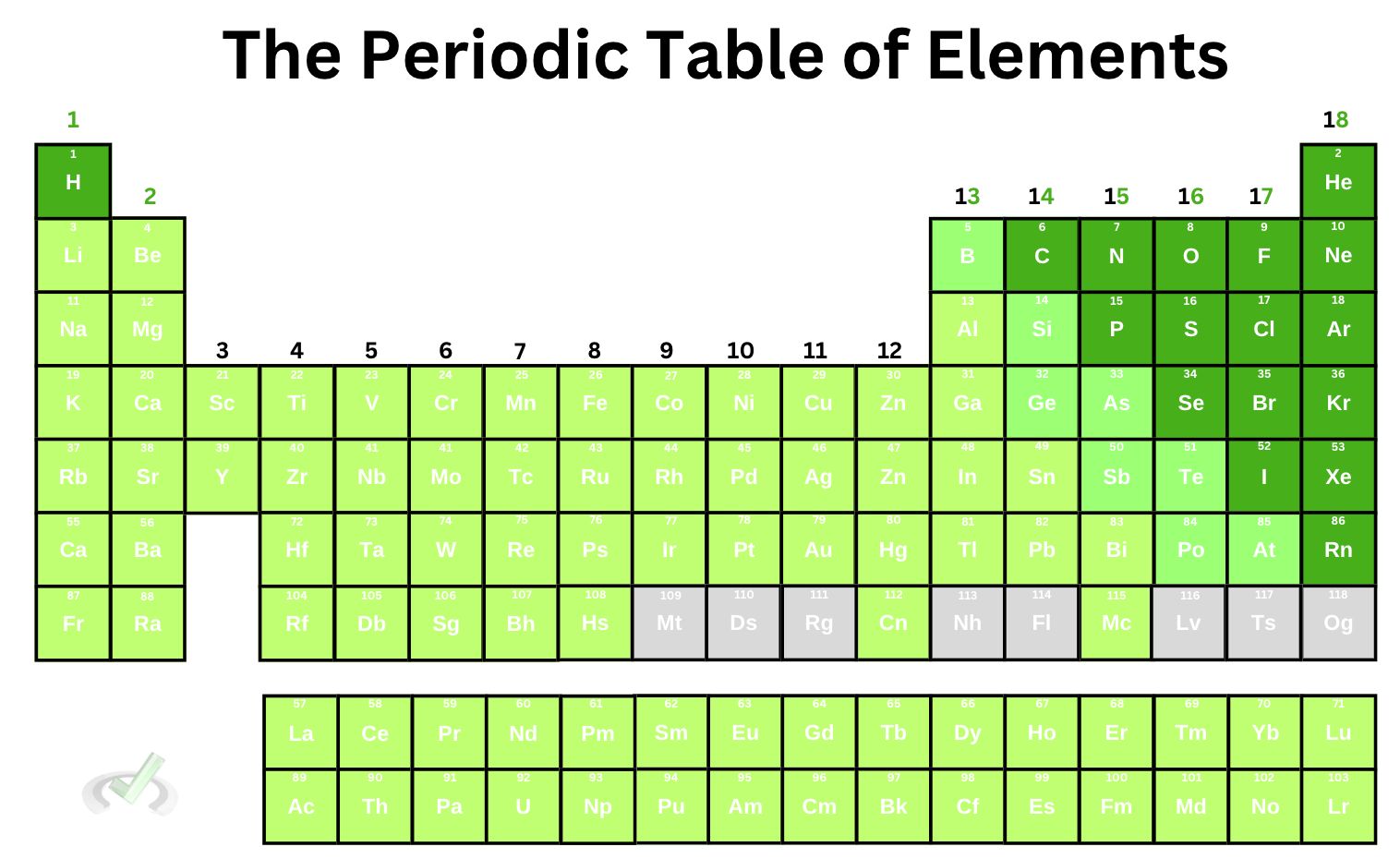
The table of elements has an odd-looking shape. You'll notice that it comes in different colors and has many numbers within each tile. Now, we know that the whole number on top of the element symbol is the element's atomic number. It tells us how many protons there are in an atom. This value is unique to an element–no two elements can share the same atomic number.
The atomic mass is the number you see at the bottom of the element, which is usually larger than the atomic number. It tells us how many protons and neutrons are in the nucleus.
The atomic number increases by 1 as we move across the periodic table. Once we reach the end, the count continues at the leftmost area at the bottom of the element we start with. The count from hydrogen and helium continues at the bottom of hydrogen for lithium; the same goes for the other elements.
In the same way, the atomic mass also increases as we move across and down the periodic table, which means that the element gets bigger and heavier.
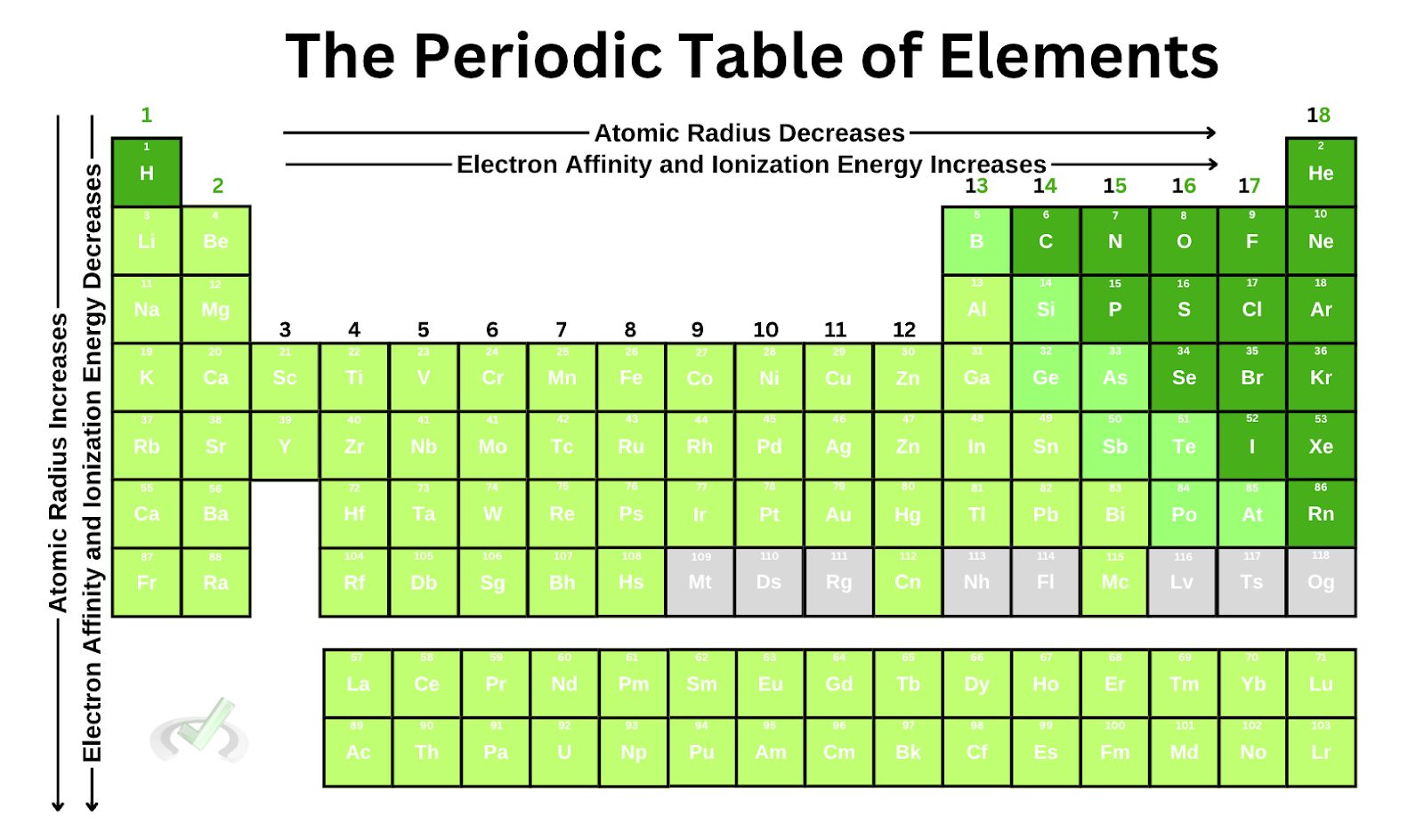
II. Atomic Radius
Now that we know how the periodic table of elements is arranged, let's look at the periodic table trends to better understand how elements look.
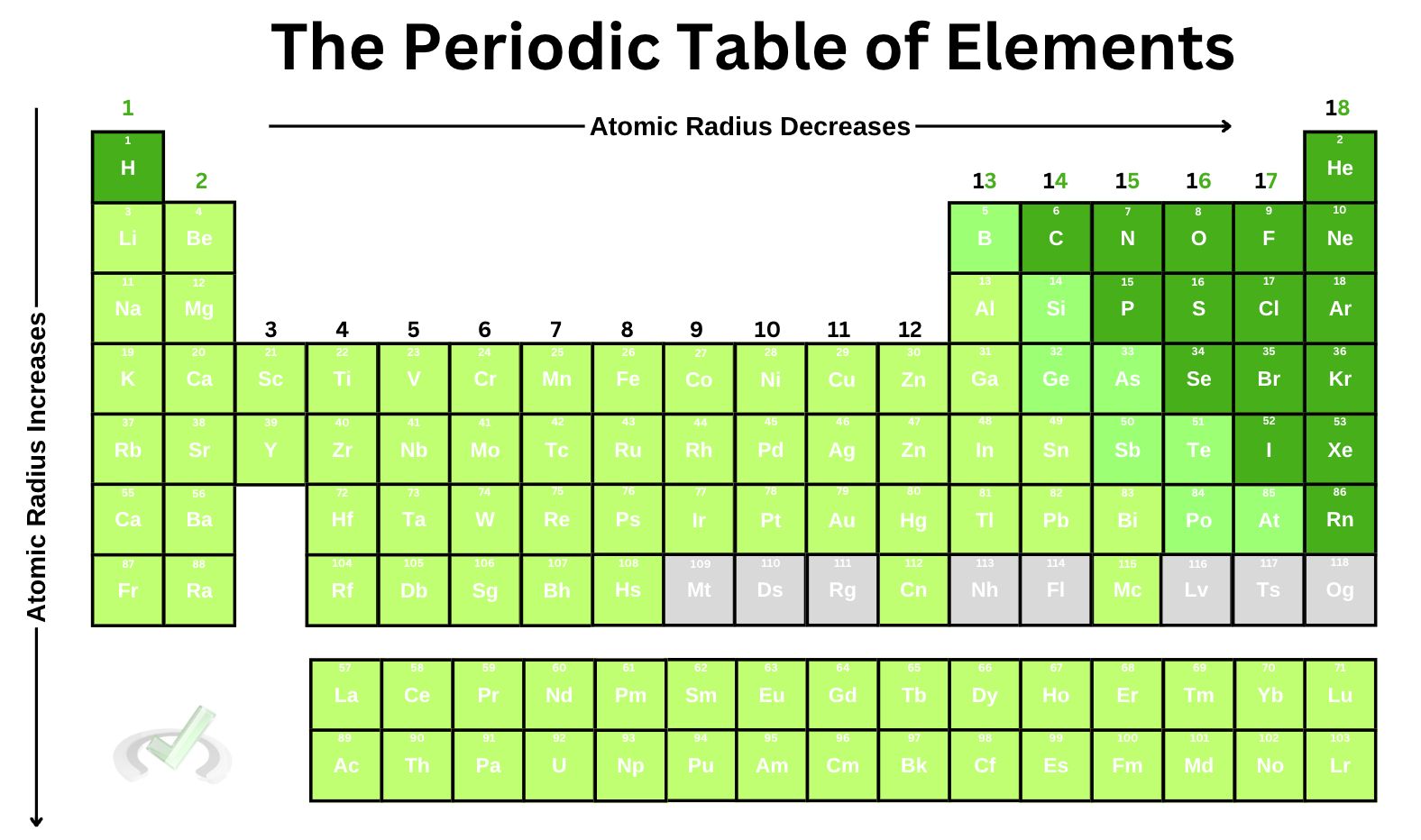
1. Atomic radius decreases as we move across the periodic table.
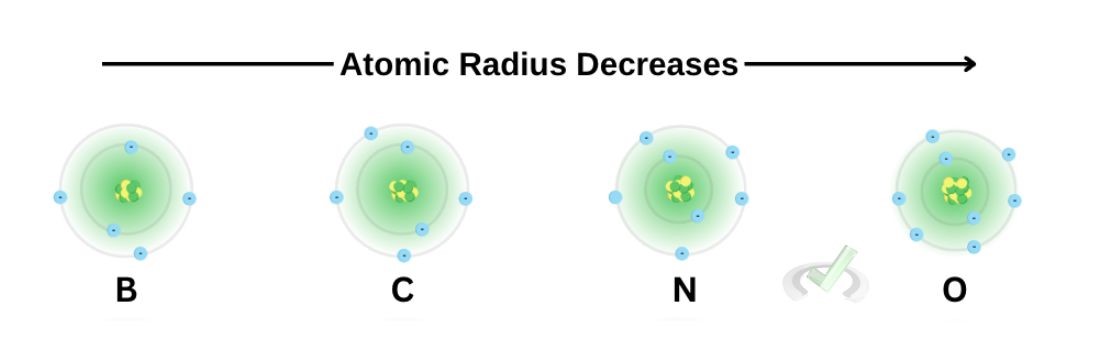
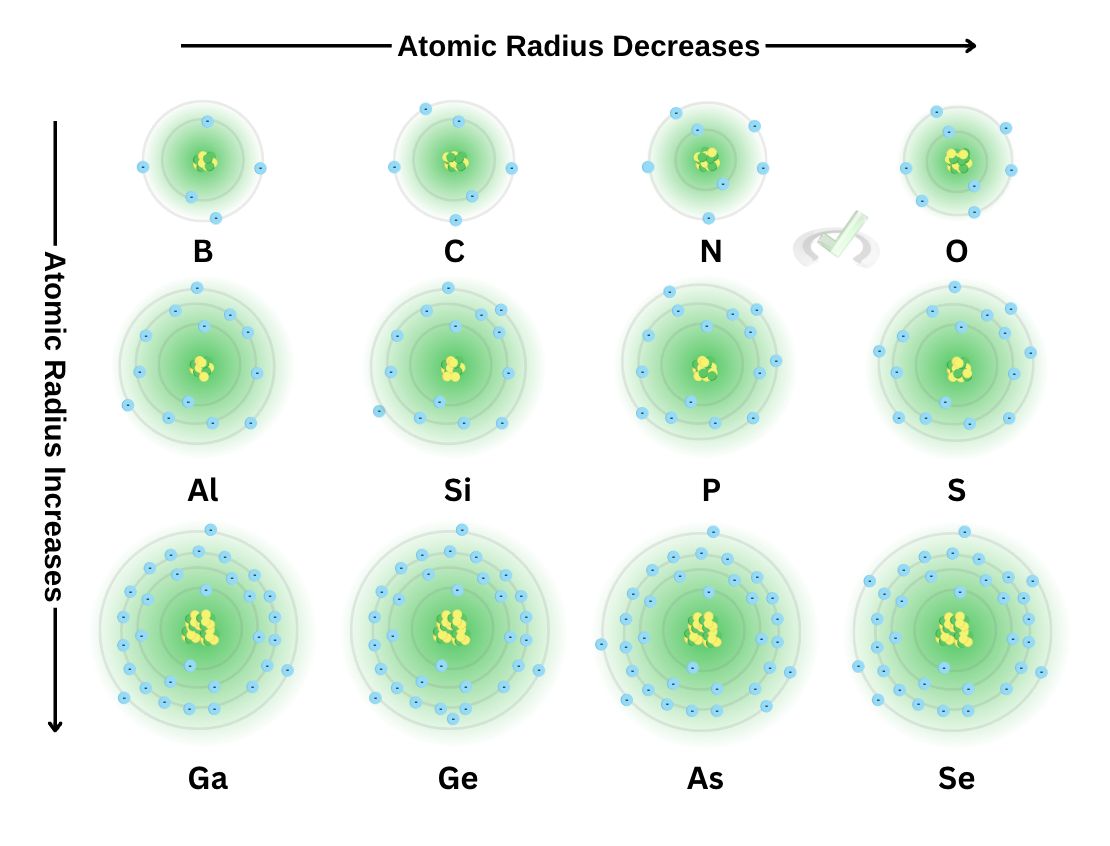
We previously discussed that the effective nuclear charge increases as we move across the periodic table from left to right. Here, we see the consecutive elements boron, carbon, nitrogen, and oxygen and their atomic model. Notice how the number of electrons and protons increases as we move across each element.
While this may seem ironic, think of it this way: Since the pull of the electrons (effective nuclear charge) increases, the more we move across the periodic table, the greater the tendency for electrons to draw nearer the nucleus.
If we look at the boron atom, we see that it has 5 electrons and 5 protons. Its Zeff is equal to three (Zeff = protons - inner core electrons). Oxygen has eight electrons. Its Zeff is six. This means the pull at electrons is stronger, making the oxygen atom more dense than boron.
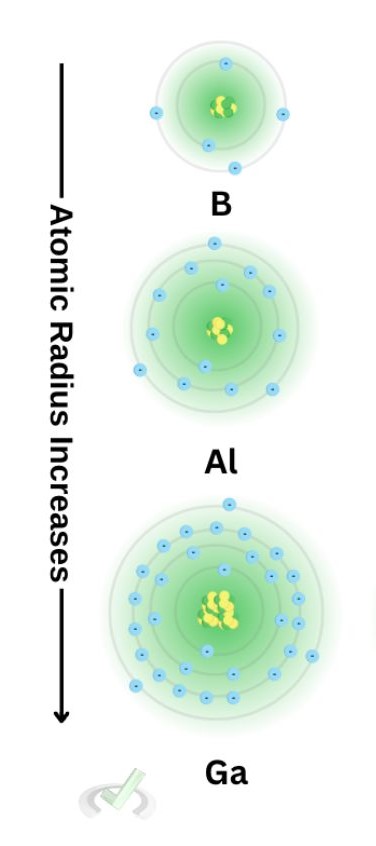
2. Atomic radius increases as we move down the periodic table.
Let's now look at some of the elements down the boron group. Here, you'll notice a few things: they all share the same number of valence electrons, the principal quantum number increases, and the atom looks significantly more prominent
All of these contribute to the fact that the atomic radius increases as we move down a group. Notice how all the orbitals we add are filled with electrons as we add an orbital. Since Zeff is also constant down a group, there is no considerable force compressing these electrons.
III. Electron Affinity and Ionization Energy
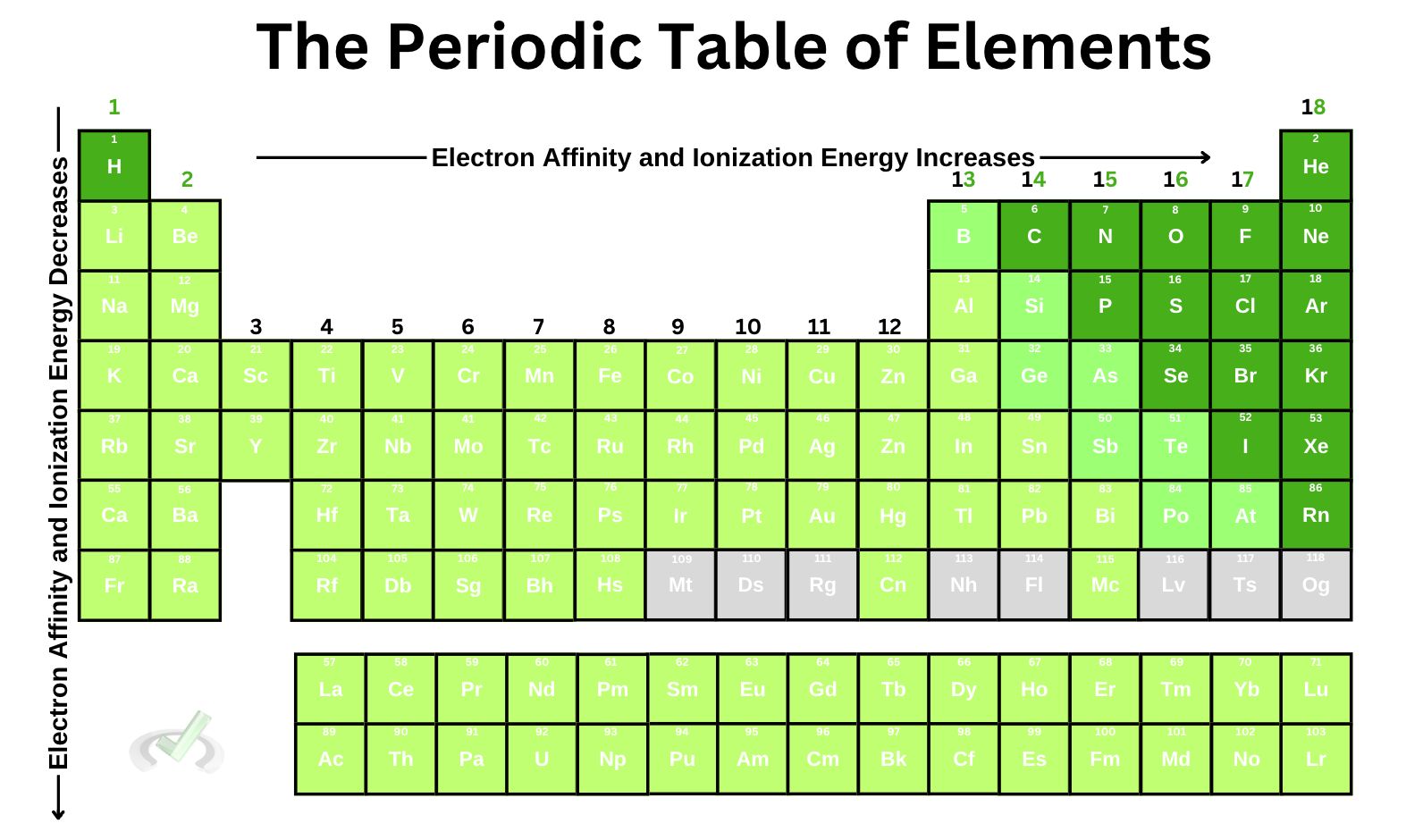
Electron affinity reflects an atom's tendency to gain an electron. This quantity tells us the change in energy that occurs when an electron is added to a neutral gaseous atom. The higher the electron affinity, the more likely an atom is to gain an electron.
- Electron affinity increases as we move across a period from left to right. This is due to the decrease in atomic radius as we move across the period. Remember that when we move across a period, the atomic radius and effective nuclear charge increase. When the effective nuclear charge is strong, the nucleus has a higher tendency to attract an electron to its positively charged nucleus.
- Electron affinity decreases as we move down a group from top to bottom. This is also caused by increased atomic radius. As the atomic size increases, the weaker the attraction between the positively charged nucleus and any surrounding electron, making it difficult for the atom to accept an electron.
The ionization energy defines the energy needed to remove an electron from a neutral gaseous atom. When the ionization energy is low, it is easier for an atom to remove an electron and become positively charged. When the ionization energy is high, it's harder for the atom to remove an electron.
- Like electron affinity, ionization energy is also affected by the effective nuclear charge since the electron shielding of the inner core electrons influences the attraction between the outer core electrons and the nucleus. Zeff increases as we move across a period, which means that the attractive force is stronger–as we move across a period, the ionization energy increases.
- The same thing is expected when we move down a group. Since Zeff is constant as we move down a group, the pull of the nucleus is not that strong enough to hold an electron. Ionization energy decreases as we move down a group because the attraction between the positively charged nucleus is not that strong with its valence electrons.
IV. Conclusion
Here’s a quick summary of the periodic table trends!
The effective nuclear charge influences a lot of elemental properties. Since we learned that Zeff increases as we move across a period and Zeff decreases as we move down a group, we can relate this to how elemental properties are. When Zeff is high, the attractive force between the nucleus and the valence electrons is strong–making the atomic radius much smaller, and electron affinity and ionization energy much stronger as we move across a period. When Zeff is low, the attractive force is not as strong which makes the electron affinity and the ionization energy much weaker as we move down a group. Adding more energy levels also makes atoms much bigger. As a result, as we move down a group and add a new energy level, we also increase the atomic radius.
These concepts are crucial because they relate to how each element behaves. As you learn more concepts, you'll soon see how ionization energy and electron affinity affect how a bond happens and how a compound behaves.
V. Key Terms
- Atomic Radius - the distance from the center of the atom to the valence electrons.
- Effective Nuclear Charge - the positive charge experienced by an electron as a result of the electrostatic force between the positively charged nucleus and the negatively charged electrons
- Electron Affinity - the tendency of an atom to gain an electron.
- Ionization Energy - the energy needed to remove an electron.
- Valence electrons - the electrons found at the outermost shell.
V. Practice Questions
Sample Practice Question 1
Arrange the following atoms in decreasing atomic radius: P, Ca, H, Ga, Fe.
A. P, Ga, Fe, Ca, H
B. Ga, P, Fe, Ca, H
C. H, Ca, Fe, P, Ga
D. Ga, P, Ca, Fe, H
Ans. B
Sample Practice Question 2
The following pairs of elements accurately describe the magnitude of their electron affinity and ionization energy except:
A. F > P
B. Na < Mg
C. Fr > Ca
D. Mg < Na
Ans. C







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
