The periodic table of elements is a great reference that doesn’t even look like a table at all! While we confuse these with all the terms included, these odd arrangements and colors we often see have a purpose.
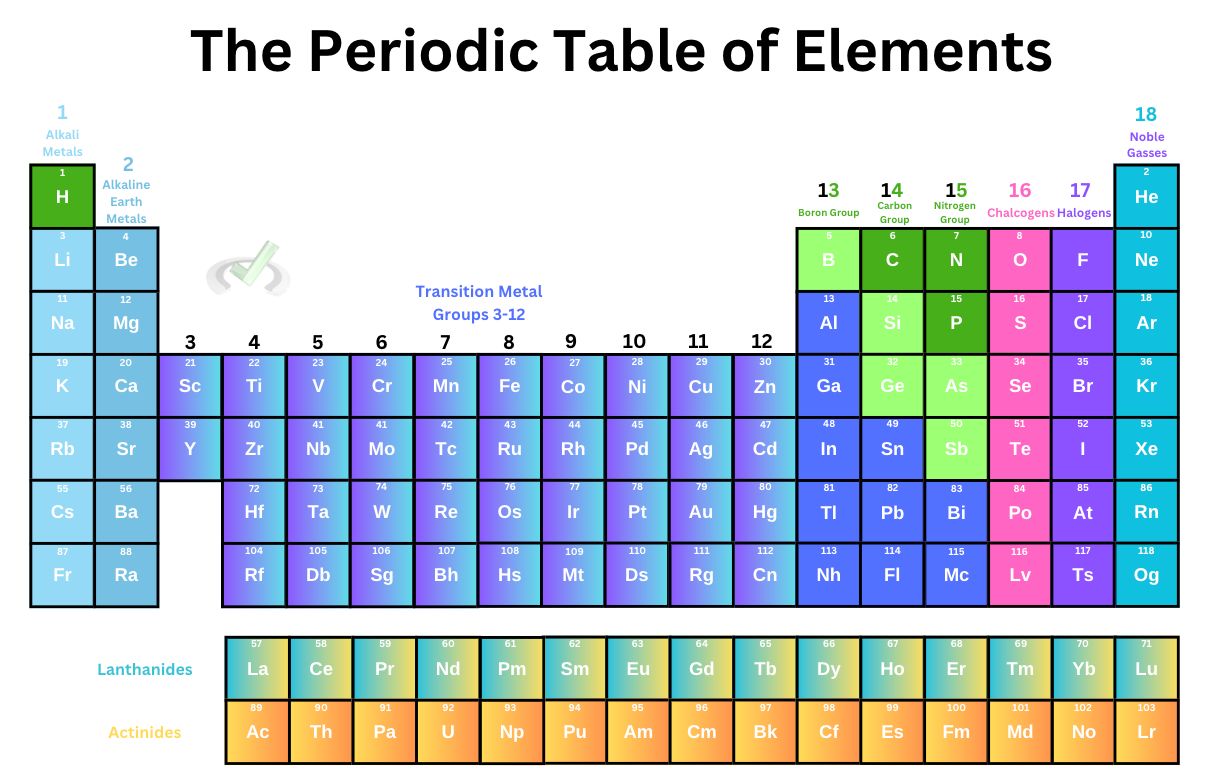
Valence Electrons
Groups 1 and 2 are known to have 1 and 2 valence electrons, respectively. Groups 13 to 17 also have 3, 4, 5, 6, and 7 valence electrons, respectively.
This should excite you because valence electrons are essential in chemical behavior. Because of these electrons, elements could form bonds and make much more complicated compounds that make up every cell in our body!
When the outer shell is completely filled, the atom can no longer take or give electrons because atoms want stability. Atoms are the most stable when they have complete valence electrons. It also follows that when an atom only has a few valence electrons, it wants to lose them to gain stability, and atoms do so by forming bonds.
In this article, we’ll see how these elements are grouped and the properties they share. We’ll also understand why valence electrons are so crucial in chemical behavior!
I. Alkali Metals and Alkaline Earth Metals
The alkali metals and alkaline metals belong under groups 1 and 2 in the periodic table, respectively. These groups have similar behavior in reactivity and their respective melting and boiling points.
A. Alkali Metals
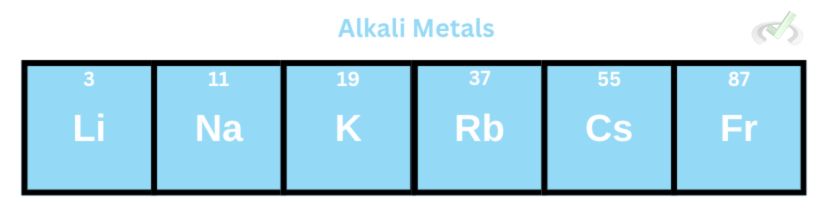
These metals are generally soft with low densities and melting points. As we move down the group, the melting and boiling point decreases.
Alkali metals are also reactive. Their reactivity increases as we move down the group. This can be explained by their valence electrons. Atoms in group 1 only have 1 valence electron. Since atoms like to have completely filled outer shells to gain stability, alkali metals readily lose their electron to be stable. These elements will also likely form cations since they can quickly lose one electron and form a cation.
They also form ionic compounds since they readily give away their valence electrons. They are usually white solids that readily dissolve in water. These metals react with water to form metal hydroxides (sodium hydroxide) and hydrogen gas. They form metal oxides (lithium oxide) when they react with oxygen.
B. Alkaline Earth Metals
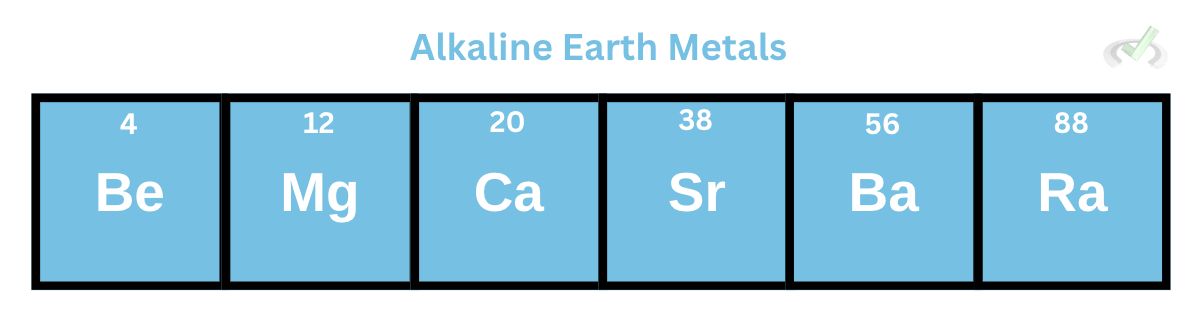
Alkaline earth metals are in the second group in the periodic table. They have two valence electrons, and similar to alkali metals, their reactivity, melting, and boiling points change as we move down the group. Since they have two electrons in the outer shell, they can lose these two electrons and form cations with a +2 charge.
These metals also get softer as we move down the group. Since more energy levels are added as we move down the group, the weaker the attractive force becomes between the nucleus and the outer shell electrons, making the electrons less tightly held.
Alkaline earth metals also become more reactive as we move down the group because the atomic radius becomes so much bigger, making it difficult for the nucleus to hold onto its valence electrons.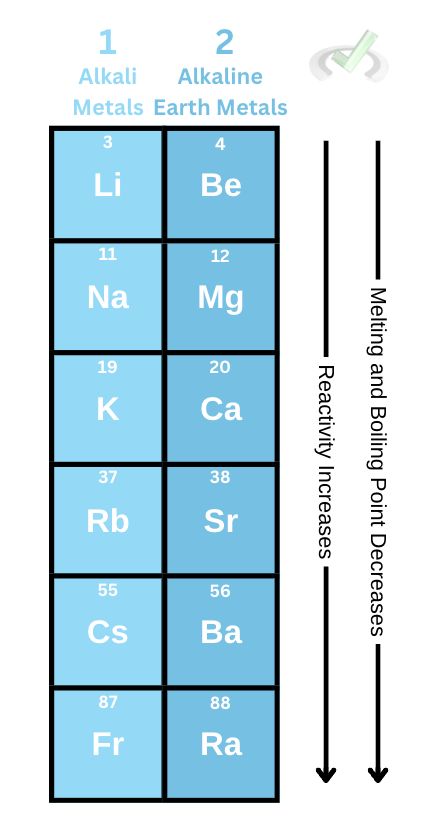
II. Chalcogens and Halogens
In our previous article, we figured that as we move across the periodic table from left to right, the ionization energy increases. Chalcogens and Halogens in the 16th and 17th periodic table groups have high ionization energies. This also means they have high electronegativity, which can attract electrons to form bonds.
A. Chalcogens
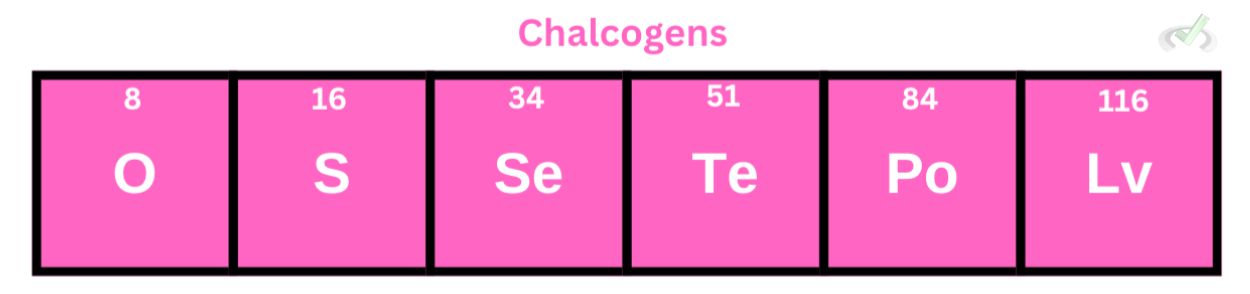
Chalcogens are under group 16 of the periodic table, they are composed of nonmetals (O, S, Se), metalloids (Te, Po), and a metal (Lv) This means that metallic character increases as we move down the group.
Oxygen, Sulfur, and Selenium are nontoxic and essential in biological systems. The other elements, though, are pretty toxic and radioactive.
These elements have six valence electrons and will only need two electrons to form a stable bond with other elements. Chalcogens will form covalent bonds–a bond among nonmetals–with other nonmetals.
It’s important to note that Polonium occurs naturally in small quantities and will rarely bond because it is unstable and rare.
B. Halogens
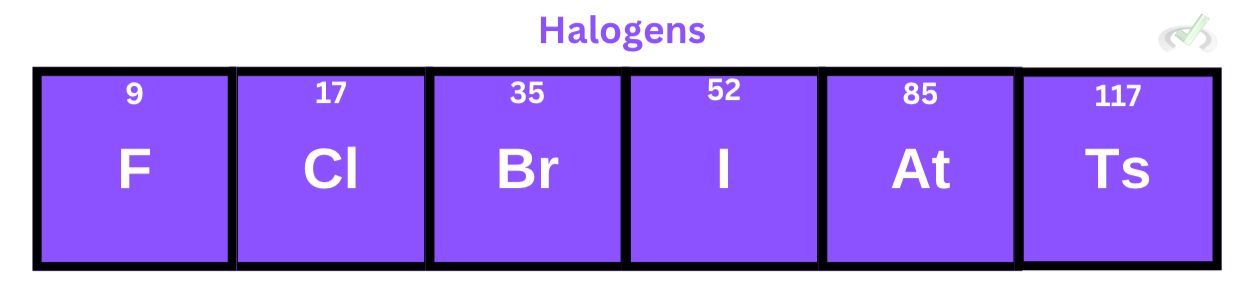
Halogens have seven valence electrons and readily accept or share one electron to form a bond. These elements can be quite dangerous in various forms. Iodine has a poisonous vapor, bromine is also poisonous in liquid form, and chlorine and fluorine are also toxic in gaseous forms. These atoms can also be diatomic, meaning they can form a bond between themselves (F2, Cl2, Br2, I2).
They usually form ionic bonds with alkali metals and covalent bonds with other nonmetals. We’ll discuss these bonds more as we move on to other articles.
We can have this general trend for chalcogens and halogens:
Reactivity decreases down a group since the atomic radius gets bigger. The attractive force between the nucleus and other electrons will not be as strong.
Melting and boiling point increases down a group since larger molecules have larger intermolecular forces, making it more difficult to boil or melt.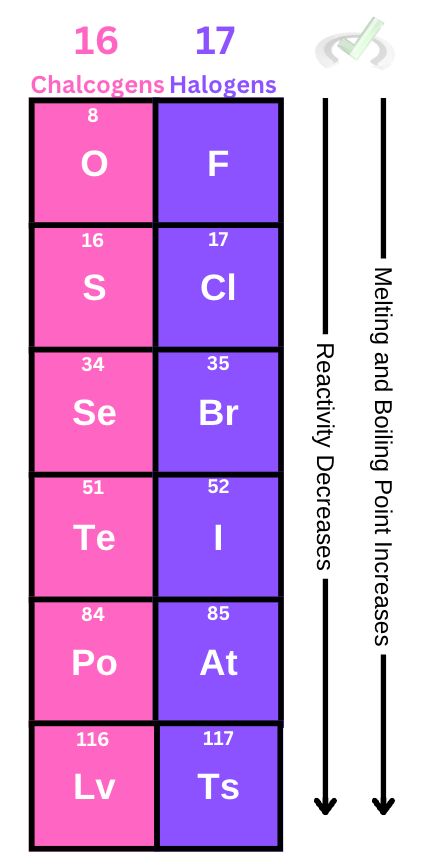
III. Noble Gasses
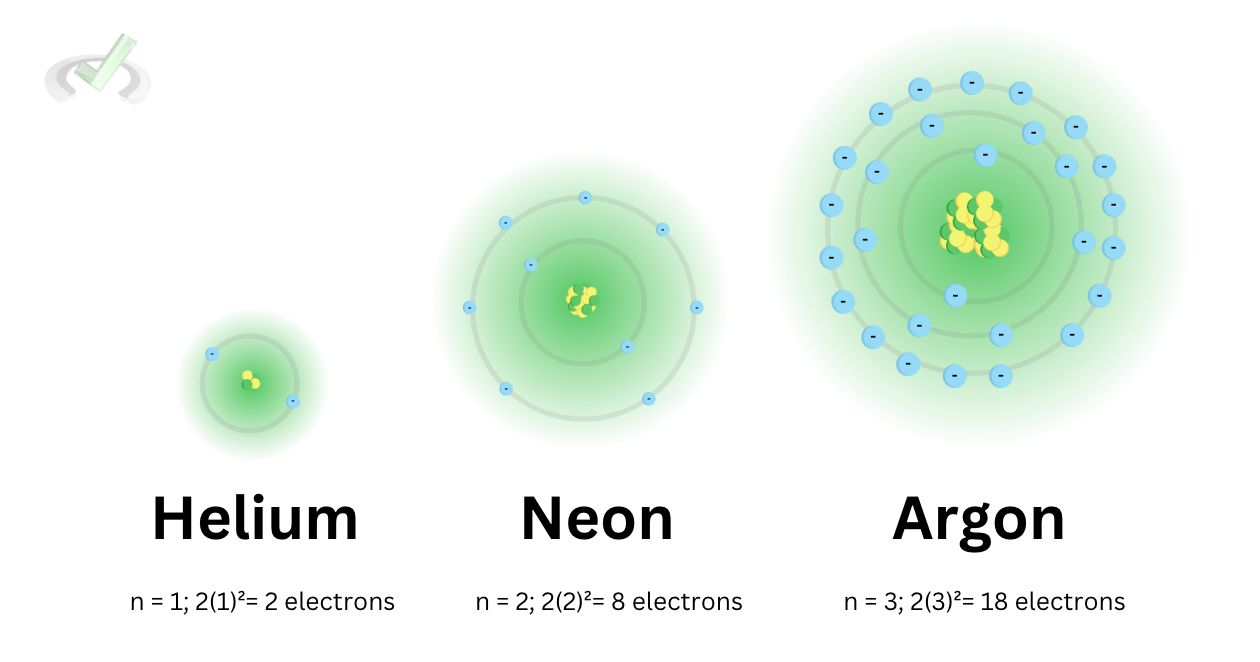
Noble gasses are just like their names–they are so noble that they do not feel the need to bond with other atoms! These gasses are in the 18th group of the periodic table. All elements under group 18 have a completely filled outer shell.
Helium has a complete outer shell with 2 electrons on the first energy level. Neon has 8 electrons on the second level, completing the outer shell. Argon also has a completely filled third energy level. The same pattern applies to the other elements under this group. Since their outer shells are completely filled, they neither have to give up electrons nor take electrons to be stable since they are already stable by themselves.
This stability makes them inert or inactive. They are colorless and non-flammable. Their boiling points increase as we move down the group.
IV. Transition Metals
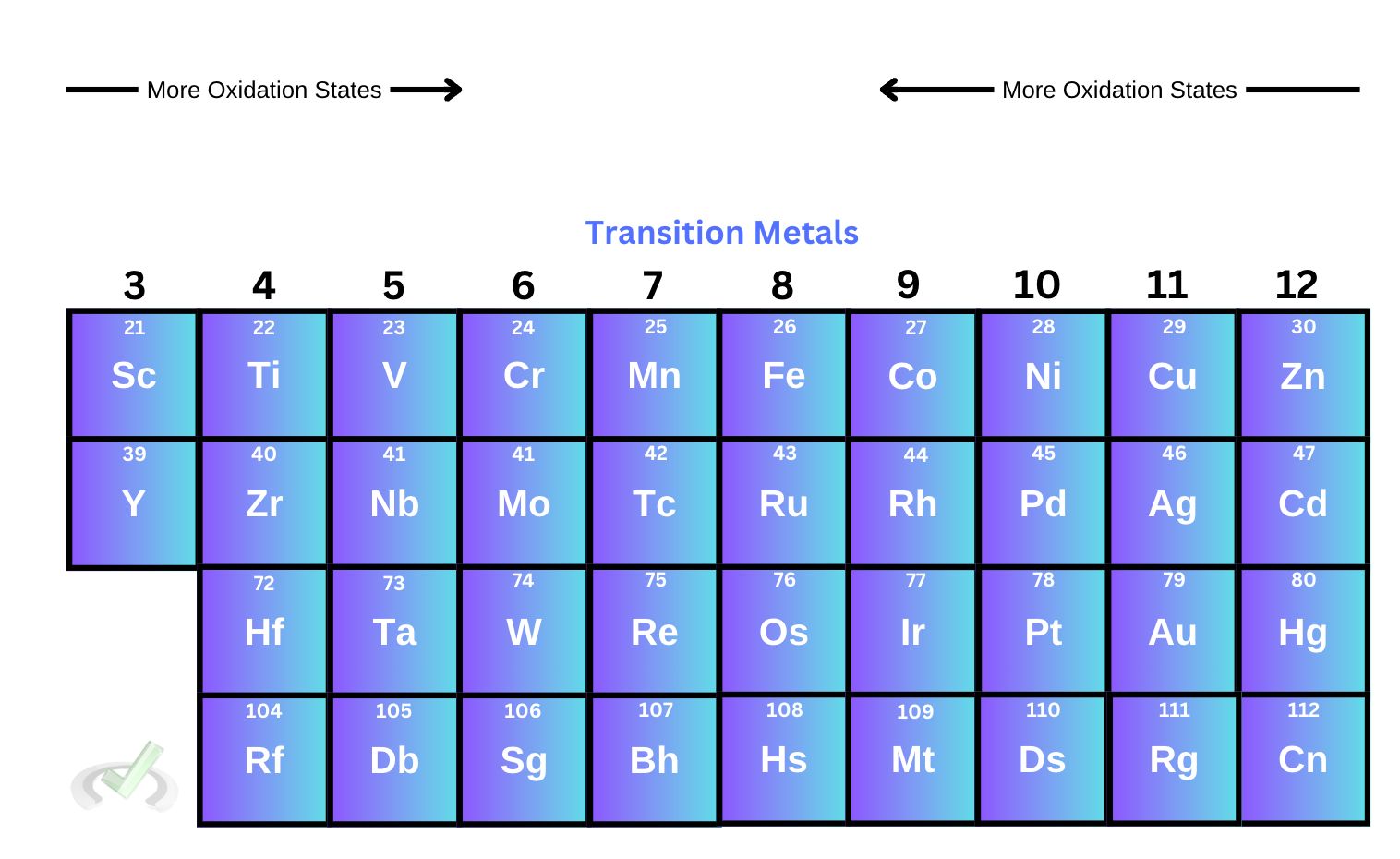
Transition metals are elements in groups 3-12 of the periodic table. They are also known to belong in the d block since it’s the subshell that’s being filled for all transition metals.
One property common to these metals is their different oxidation states. Since the valence electrons are in the d subshell, the effective nuclear charge, or the pull the nucleus has on its electrons, is not as strong. This means that the atom can easily lose electrons and form different cations. Some transition metals, such as copper, are also more stable as ions.
They have high melting and boiling points and are good conductors of heat and electricity. The metals we use for our wires are usually made of copper since electrons move freely among metals.
A trend that is not as reliable for transition metals is when we move towards the center of the d block, elements tend to have more oxidation states. Elements found at the center, such as manganese and iron, have a wider range of oxidation states. This trend, though, should be taken with a grain of salt. Some elements found on the left side of the d block can have more oxidation states than elements near the center.
V. Conclusion
The periodic table of elements can almost seem like a perfect map for elements. The early creators of the periodic table left out spaces for other elements that were yet to be discovered and yet future generations eventually figured out the elements that go into these slots!
The modern day periodic table of elements is arranged neatly–-somehow we found a way to arrange electrons with the same number of valence electrons and relate it to their behavior! We can summarize everything we learned using the following points:
- Alkali metals (Group 1) and alkaline earth metals (Group 2) exhibit similar properties due to their valence electrons. As we move down the group, they become more reactive, and their melting and boiling points decrease.
- Chalcogens (Group 16) and Halogens (Group 17) also exhibit similar properties. Their melting and boiling points increase and their reactivity decreases as we move down the group.
- Transition metals have a variety of properties but one common property that they have is that they have multiple oxidation states and high melting and boiling points.
VI. Key Terms
- Alkali Metals - Reactive metals in group 1 with 1 valence electron.
- Alkaline Earth Metals - Reactive metals in group 2 with 2 valence electrons.
- Chalcogens - Elements under group 16 with six valence electrons. These are composed of nonmetals, metalloids, and metals.
- Effective Nuclear Charge - The positive charge experienced by electrons (usually measured for electrons in the outer shell).
- Halogens - Reactive nonmetals in group 17 of the periodic table with 7 valence electrons.
- Noble Gasses - Inert elements with completely filled energy levels.
- Transition Metals - Elements found in groups 3-12 of the periodic table.
VII. Practice Question
Sample Practice Question 1
Which of the following is not likely to be true?
A. Halogens have 7 valence electrons, which means they readily give up 7 electrons to form a bond.
B. Chalcogens have 6 valence electrons, which means they readily accept 2 electrons to form a bond.
C. Alkali metals can form a bond by donating an electron.
D. Alkaline earth metals can donate 2 electrons to form a bond.
Ans. A
Sample Practice Question 2
All of the following statements are true except:
A. Noble gasses do not form a bond with other elements.
B. Halogens can be toxic in various forms.
C. Metallic character increases as we move up the group for chalcogens.
D. Noble gasses are inert.
Ans. C







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
