What’s the thing that connects your Nestle lemon flavor iced tea, the Starbucks classic syrup, and the boiling salt water that makes your pasta? As you probably guessed by the title of this chapter overview, they are all examples of solutions.
While we usually think of solutions as merely something dissolving into something else, there are definitely — as with all other topics on the MCAT — other complexities involved with the formation and nature of solutions.
This chapter overview will introduce you to some of the basic terms and concepts associated with solutions. Let’s get started!
Solutions on the MCAT: What You Need to Know
Topics on general chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
We’ve calculated that there can be 5-7 possible questions that will cover solutions, concepts, and calculations.
Introductory general chemistry accounts for 30% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Solution
The great thing about studying and reviewing solutions is that you’ll apply a lot of other topics from other general chemistry topics!
From reaction equilibrium, basic stoichiometry, chemical bonding (and many more!), the concept of solutions takes a little bit from each topic and combines them together as we’ll see as we’ll go into the chapter overview!
1. Main Terms and Units of Solution
In order to make a solution, only 2 components are needed: the solute and the solvent! The solute is the component that’s dissolved into the solvent. Conversely, the solvent is the component of the solution that dissolves the solute.
Almost always, solutions involve a solid solute being dissolved into a liquid solvent. However, there are cases where a solution can be created by a gas solute and gas solvent!
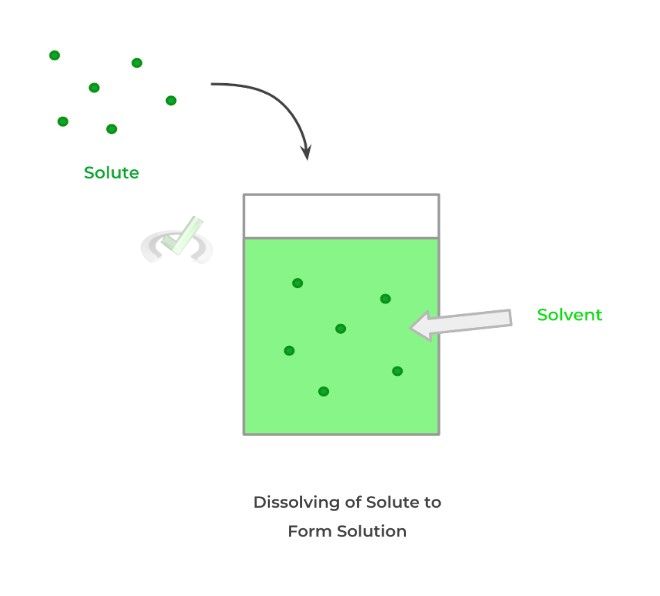
There are some units we can use in order to put a number to how concentrated a solution is. As shown below, 2 important values that quantify concentration are molarity and molality!
- Molarity (M): Ratio of the moles of solute to the liters of solution
- Molality (m): Ratio of the moles of solute to the kilograms of solvent

You’ll without a doubt come across molarity significantly more during the MCAT compared to molality, but it also doesn’t hurt to have this other calculation in mind! Note the difference in the denominators: molarity uses liters of solution, while molality utilizes kilograms of solvent.
One property of aqueous solutions (i.e. solutions that use water as the solvent) is that they can lower their concentration via the addition of more water — this process is called a dilution.
This makes sense because the addition of more water increases the denominator value in the molarity equation, lowering the value. We can actually use the equation below in order to solve how much water needs to be added to attain the new molarity!
Notice how both the products on each side of the equation (M1*V1 and M2*V2) are equal to the moles of solute! It’s important to understand that despite the changes in concentration, the value for the moles of solute remains constant!
In order to find the amount of water that needs to be added to achieve the final, diluted molarity, subtract the final volume from the initial volume — this will equal the amount of water that needs to be added!
Full Study Notes : Main Terms and Units of Solutions
For more in-depth content review for a little more on the main terms, units, and equations utilized for solutions, check out these detailed lesson notes created by top MCAT scorers.
2. Basic Solubility Rules and Concepts
There are a couple of general rules and principles regarding the solubility of substances into aqueous solutions. Solubility simply refers to the ability of a solute to dissolve into a solvent to form a solution.
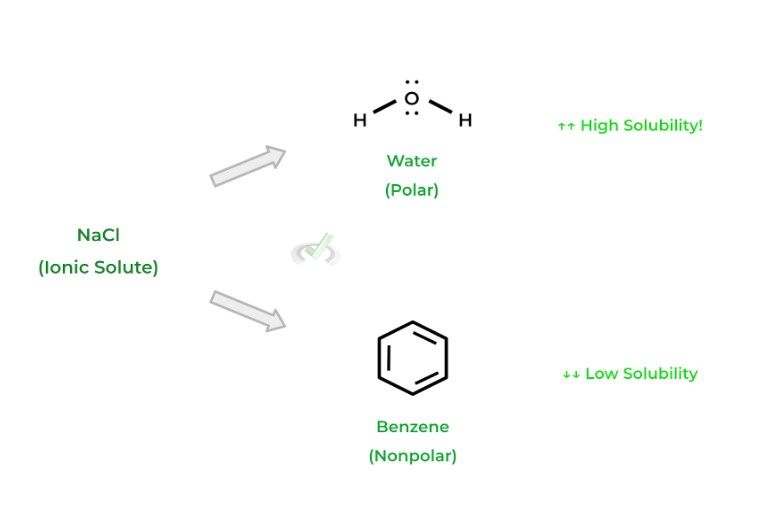
One important generality for solubility is the “like dissolves like” rule of thumb, which heavily refers to polar and nonpolar molecules. As the name implies, polar/ionic solutes will dissolve well in polar solvents, while nonpolar solutes will dissolve well in nonpolar solvents!
This is due to the favorable intermolecular interactions that occur between “like” molecules! Likewise, the converse of the above relationships is true: mixing polar solutes into a nonpolar solvent (and vice versa) results in low solubility of the solute.A. High Solubility
Polar Solute and Polar Solvent
Ionic Solute and Polar Solvent
Nonpolar Solute and Nonpolar Solvent
B. Low Solubility
Polar Solute and Nonpolar Solvent (and vice versa)
Ionic Solute and Nonpolar Solvent
You’ll come back across this topic when covering various chromatography techniques used in the laboratory, as covered in our organic chemistry module. Have a look at the diagram below to get a better visual understanding of the “like dissolves like” rule of thumb!
In addition to this, there are a couple more specific general solubility rules in regard to ionic salts being dissolved in water. There are actually a lot more rules and exceptions, but the ones covered below should suffice in your MCAT prep!
1. Group 1 alkali metals and ammonium (NH4+) salts are water soluble.
2. Acetate (CH3COO-) and nitrate (NO3-) salts are water soluble.
3. Mercury, lead, and silver salts are insoluble in water.
An important thing to note about these rules is that they are ranked in a hierarchical order! In this case, rules 1 and 2 have higher priority than rule 3. Look at the example below!
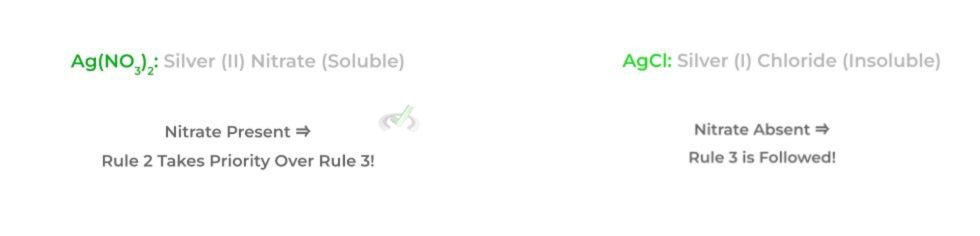
Full Study Notes : Basic Solubility Rules
For more in-depth content review on the main solubility rules and principles, check out these detailed lesson notes created by top MCAT scorers.
3. Equilibrium of Solutions
Interestingly, concepts of chemical equilibrium can also be applied to solutions, specifically in the dissolving of solutes! In the case of solutions, equilibrium refers to when the maximum concentration of a solute is dissolved.
One way to think of this is that only so much of a substance can be dissolved! At a certain point, the addition of more solute won’t dissolve further — the solute will simply remain as a solid (Note: We’ll cover the exception of supersaturated solutions).
Specifically, we use the term saturated to describe a solution at this equilibrium where no more solute can be dissolved. Similar to the equilibrium constant, we can also put a number to solution equilibrium. Let’s look at the equation and an example below!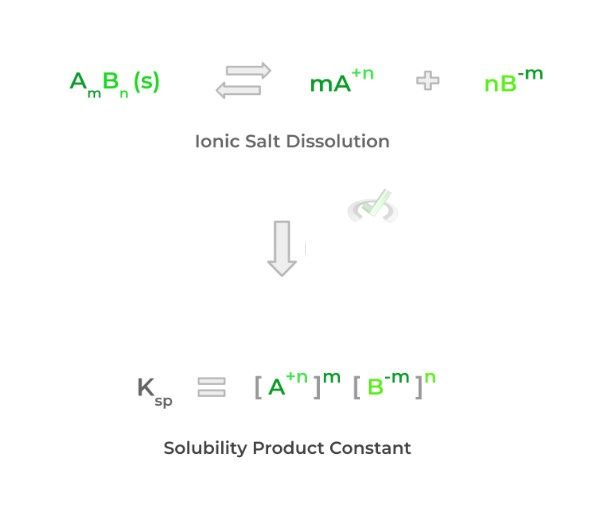
9 times out of 10, solution equilibria deal with the dissolving of an ionic compound in water. Take this time also to brush up on how to form ionic chemical formulas from ions — this will help to understand the equation above a little better!
For the most part, we calculate the solubility product constant and equilibrium constant the same way; however, notice how the ionic solid reactant is not included. This is because we don’t include solid or liquid reactants or products when calculating the equilibrium constant!
A lot can be concluded from the Ksp value of a specific ionic compound: the higher the Ksp value, the more likely the ionic compound will dissolve (high solubility); conversely, the lower the Ksp value, the less likely the ionic compound will dissolve (low solubility). Let’s use the example of barium nitrate and silver (I) chloride.
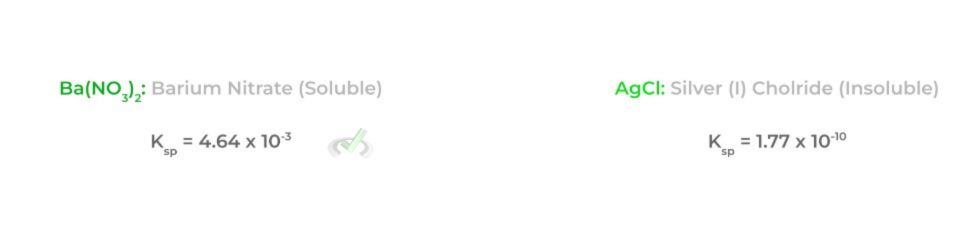
In addition to the Ksp value, we also have a number called the ion product (IP), which is essentially analogous to the reaction quotient of a chemical reaction! Similarly, we can also make various conclusions when comparing the value of the ion product to the Ksp as shown below:
A. IP < Ksp: Unsaturated → Solute Will Continue to Dissolve
>> Why?
- The solution hasn’t reached saturation yet (i.e. when the maximum amount of solute has been dissolved). Because of this, the solute will continue to dissolve.
B. IP = Ksp: Saturated → Equilibrium Achieved
>> Why?
- The solution is now at a point where the maximum amount of solute has been dissolved. When more solute is added, the solute will remain as a solid.
- There is an exception for supersaturated solutions, which is discussed in the next point.
C. IP > Ksp: Supersaturated → Any Disturbance Causes Precipitation of Solute
>> Why?
- Supersaturated solutions have more than the maximum amount of solute dissolved. This can be achieved by heating the solution to high temperatures, adding more solute, and then slowly cooling it.
- At this point, the supersaturated solution is very delicate and any disturbance will result in the precipitation of solute back into a solid.
Full Study Notes : Equilibrium of Solutions
For more in-depth content review on equilibrium of solutions, check out these detailed lesson notes created by top MCAT scorers.
4. The Common Ion Effect
While temperature and the Ksp are key factors in determining how much of a solute will dissolve to make a solution, another key principle that affects how much solute will dissolve is the common ion effect.
Essentially, this is just another application of Le Chatelier’s principle for solutions! If there’s already an ion present in the solution that’s also present in the incoming salt (hence, the term “common ion”), the amount of salt that will be dissolved is reduced. Take a look at the example below!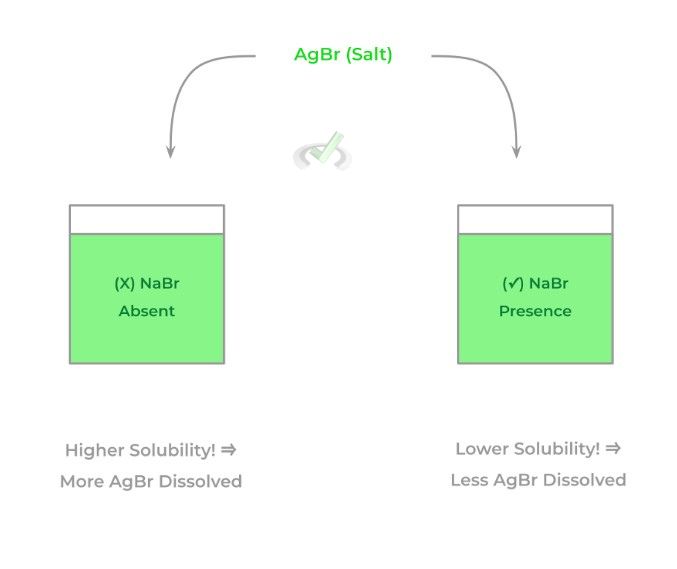
In the right solution, because there’s already a common ion present in the form of bromide, less silver bromide will be dissolved as a result — again, this is just another application of Le Chatelier’s principle in the context of solutions!
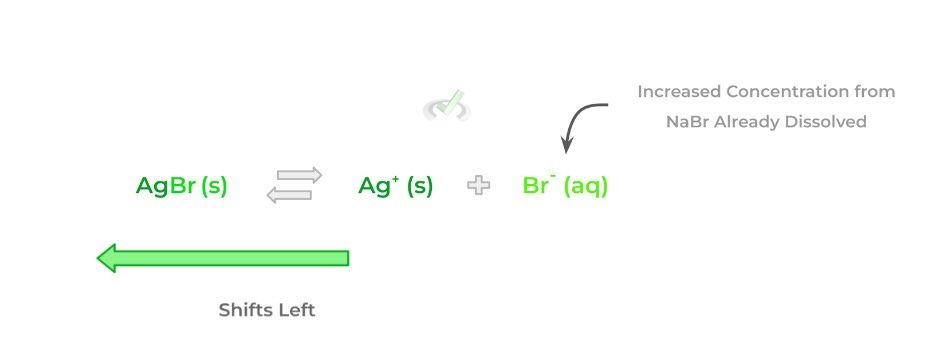
In terms of calculation questions that might appear on the MCAT, those involving the calculations with the common ion effect are a bit more complicated. We cover more about the common ion effect as well as strategies to approach the calculations in this article!
Full Study Notes : The Common Ion Effect
For more in-depth content review on the common ion effect, check out these detailed lesson notes created by top MCAT scorers.
5. Colligative Properties
The term “colligative properties” really just refers to the various characteristics that solutions display as a result of the dissolved solutes! While there are many different colligative properties to note, we’ll cover 3 main ones — note that the colligative properties that occur are a result of increased solute concentration!
A. Vapor Pressure Depression
>> Why?
Recall that vapor pressure is the pressure that’s exerted by the evaporated solution vapor on the surface of the liquid solution.
When a higher concentration of solutes is dissolved, the solutes prevent the amount of solvent that can be evaporated, decreasing the vapor pressure. Think of it like a “shield” or “wall” which prevents the solvent molecules from evaporating!
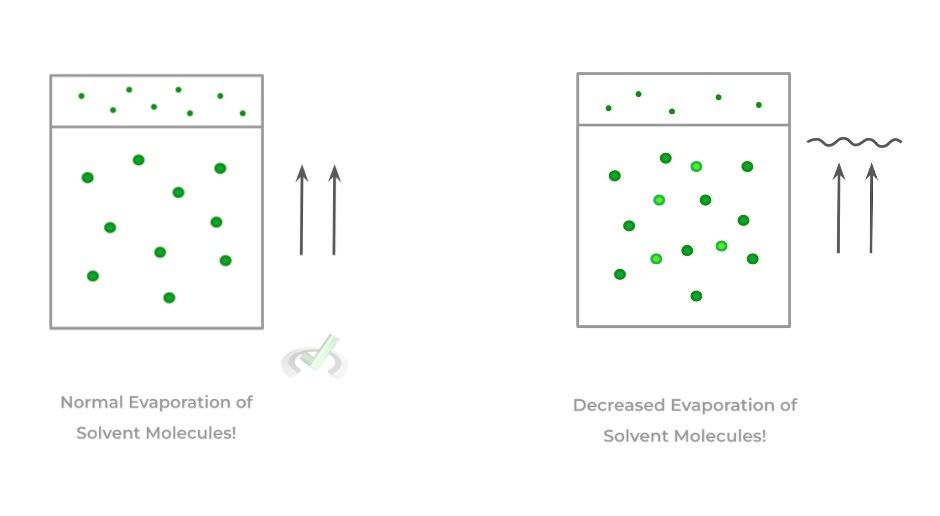
B. Boiling Point Elevation
>> Why?
To understand the rationale behind this, we have to go back to the above colligative property — the more solute dissolved, the lower the vapor pressure.
Recall also that a solution boils when its vapor pressure equals the external pressure. Because increased solute concentration results in a lower vapor pressure, and thus results in a higher temperature needed for the solution to boil. Follow the reasoning below!

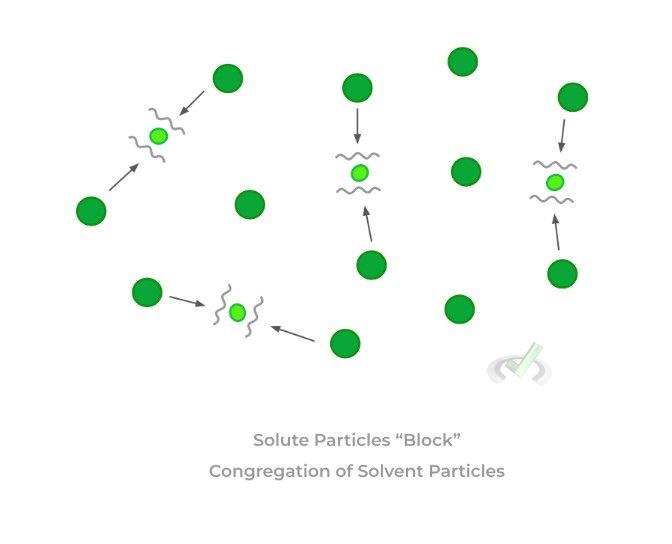
C. Freezing Point Depression
>> Why?
In order to form a solid, the solvent molecules must condense and congregate to form the solid structure. However, when more solute is dissolved into the solution, this congregation is disrupted!
It’s kinda like trying to compress a spring with crayons trapped within the wedges — those crayons make it harder to compress the spring, just like the solute particles make it harder for the solvent particles to condense.
Full Study Notes : Colligative Properties
For more in-depth content review on colligative properties, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about solutions in general chemistry!
Term | Definition |
|---|---|
Solute | Component of the solution which dissolves in the solvent |
Solvent | Component of the solution which dissolves the incoming solutes |
Molarity | Ratio of the moles of solute to the liters of solution |
Molality | Ratio of the moles of solute to the kilograms of solvent |
Dilution | A method to decrease the concentration of an aqueous solution by the addition of more water |
Saturated | Term to describe a solution at solubility equilibrium; At this point, the maximum amount of solute is dissolved in the solvent |
Unsaturated | Term to describe a solution not yet at solubility equilibrium; Because the maximum amount of solute has not been dissolved, more solute will be dissolved until equilibrium is reached |
Supersaturated | Term to describe a solution past solubility equilibrium; It’s possible to create these solutions by heating the solution to very high temperatures, adding more solute, and then slowly cooling it down |
Common Ion Effect | Application of Le Chatelier’s principle in solution equilibria where the presence of a common ion in a solution decreases the solubility of an incoming salt |
Colligative Properties | Properties of solutions which are dependent on the amount of solute particles that are dissolved! |
Additional FAQs - Solutions on the MCAT
What are the Main Solubility Rules? – MCAT
In addition, there are 3 main solubility rules we can apply to salts to see if they’ll be soluble in water: 1) group 1 alkali metal and ammonium salts are water soluble, 2) acetate and nitrate salts are water soluble, and 3) mercury, lead, and silver salts are insoluble. Note, that the rules are listed in hierarchical order, where rules 1 and 2 take priority over rule 3!
What is Normality? – MCAT
Because there are 3 equivalents of hydrogen (as indicated by the “3” subscript, we just multiply the molarity of the entire solute by the 3 equivalents to get the normality; hence, the hydrogen has a normality of 0.3 M when the phosphoric acid dissociates.
What is an Ideal Gas — MCAT?
Is the Ideal Gas Constant on the MCAT?
Additional Reading Links – Study Notes for Solutions on the MCAT
Additional Reading: General Chemistry MCAT Topics:
- Atomic Structure on the MCAT
- Periodic Table on the MCAT
- Bonding and Chemical Reactions on the MCAT
- Chemical Kinetics on the MCAT
- Electrochemistry on the MCAT
- Equilibrium on the MCAT
- Acids and Bases on the MCAT
- Stoichiometry on the MCAT
- The Gas Phase on the MCAT
- Thermochemistry on the MCAT
- Redox Reactions General Chemistry MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
