Colligative properties depend on the number of solute particles in a solution, not their nature. These properties affect the physical properties of solutions, such as boiling and freezing points. Understanding colligative properties is essential in chemistry, biology, and environmental science.
I. Introduction to Colligative Properties
Colligative properties are unique because they depend on the concentration of solute particles in a solution. There are four main colligative properties: boiling point elevation, freezing point depression, vapor pressure lowering, and osmotic pressure.
Boiling Point Elevation
When a non-volatile solute is added to a solvent, the boiling point of the solution increases. This is because the solute particles disrupt the solvent molecules, making it harder for them to escape into the gas phase.
For example, when salt (NaCl) is added to water, it dissociates into sodium (Na⁺) and chloride (Cl⁻) ions. These ions interfere with the hydrogen bonding among water molecules. As a result, more heat is needed to make the water boil.
The equation to calculate the boiling point elevation is:
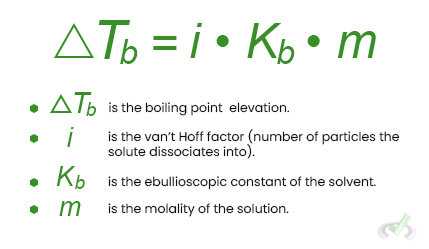
Freezing Point Depression
Adding a solute to a solvent lowers the freezing point of the solution. This happens because the solute particles interfere with the formation of the solid phase of the solvent.
For instance, when salt (NaCl) is added to ice, it dissolves into Na⁺ and Cl⁻ ions. These ions disrupt the ice's orderly structure, causing it to melt at a lower temperature.
The equation to calculate the freezing point depression is:
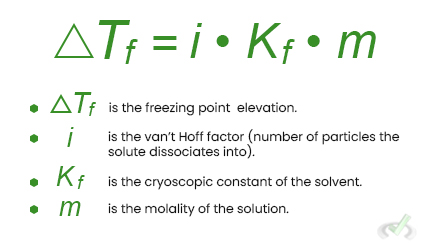
Vapor Pressure Lowering
Adding a non-volatile solute to a solvent decreases the solution's vapor pressure. This is because fewer solvent molecules can escape into the gas phase due to the presence of solute particles.
Raoult's law explains this phenomenon:
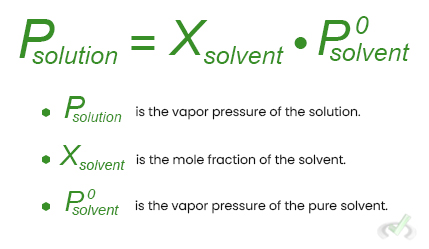
Osmotic Pressure
Osmotic pressure is the pressure required to stop the flow of solvent molecules through a semipermeable membrane from a dilute solution to a concentrated one.
The equation for osmotic pressure is:

II. Examples of Colligative Properties
Colligative properties have practical implications in everyday life and industrial applications. Here are some examples to illustrate how these properties are used.
Boiling Point Elevation in Everyday Life
When you add salt to water for cooking pasta, the boiling point of the water increases. This is because the Na⁺ and Cl⁻ ions disrupt the hydrogen bonding among water molecules, requiring more heat to reach the boiling point.
Freezing Point Depression in Road Safety
In cold regions, salt is spread on roads to prevent ice formation. This is due to the freezing point depression caused by the Na⁺ and Cl⁻ ions from the salt. These ions lower the freezing point of water, melting ice and preventing new ice from forming.
Vapor Pressure Lowering in Solutions
In a saline solution, the presence of Na⁺ and Cl⁻ ions lowers the water's vapor pressure, which is why saline solutions evaporate more slowly than pure water. The solute particles inhibit the escape of solvent molecules into the vapor phase.
Osmotic Pressure in Biological Systems
Osmotic pressure is vital in biological systems. For example, red blood cells maintain their shape due to osmotic pressure. When placed in a hypotonic solution, they swell and may burst. In a hypertonic solution, they shrink. Proper osmotic balance is crucial for cell function.
III. Practical Applications
Colligative properties are not just theoretical but are used in various industrial and environmental applications. Here are a few examples.
Industrial Applications
Colligative properties are used in various industrial processes. For instance, antifreeze solutions in car radiators rely on freezing point depression. Adding ethylene glycol to water lowers the solution's freezing point, preventing the engine from freezing in cold weather.
Environmental Science
Understanding colligative properties helps in environmental science. For example, using salt to de-ice roads can impact the environment. Excessive salt can lead to soil and water pollution, affecting plant and aquatic life.
IV. Bridge/Overlap
Colligative properties are closely linked to several broader chemistry concepts. Understanding these connections can provide a deeper insight into solutions' behavior.
Chemical Equilibrium
Colligative properties are linked to chemical equilibrium. For example, lowering vapor pressure affects the equilibrium between liquid and gas phases. Understanding this concept helps predict how solutions behave under different conditions.
Thermodynamics
Thermodynamics principles explain colligative properties. For instance, the increase in boiling point due to a solute can be understood through the second law of thermodynamics. It states that systems tend to move towards maximum entropy, which explains why solutes disrupt the orderly structure of solvents.
V. Wrap-Up and Key Terms
Conclusion
Understanding colligative properties involves mastering several key concepts and terms. These properties play a crucial role in understanding the behavior of solutions. They are applicable in everyday life, industrial processes, and biological systems. Mastering these concepts is fundamental for advanced studies in chemistry and related fields.
Key Terms
VI. Practical Questions
Sample Practice Question 1
What happens to the boiling point of water when salt is added?
A. It decreases.
B. It stays the same.
C. It increases.
D. It fluctuates.
Ans. C
Adding salt to water increases the boiling point because salt disrupts the water molecules' hydrogen bonds, requiring more heat to bring the water to a boil.
Sample Practice Question 2
Which colligative property is responsible for de-icing roads?
A. Boiling point elevation
B. Freezing point depression
C. Vapor pressure lowering
D. Osmotic pressure
Ans. B
Salt de-ices roads by lowering the freezing point of water. This prevents ice from forming, making the roads safer by keeping them liquid at lower temperatures.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
