Chemical compounds can look confusing at first glance. Some compounds seem to have subscripts on every atom, and some don’t have any subscripts at all. Before discussing a compound's empirical and chemical formula, let’s first look at one compound and identify what this tells us.

We can imply a few things from what we have above. Ammonia has nitrogen represented by its symbol, N. It also has hydrogen, represented by H. The number in front tells us how many moles of ammonia we have. Here, this means we have 2 moles of NH₃. We’re not assuming how many molecules we have; we only know that we have 2 moles of ammonia. The subscript, 3, next to hydrogen, indicates that we have 3 hydrogen atoms in ammonia. This means that there are 3 hydrogen atoms in one mole of ammonia. Nitrogen does not have a subscript since the presence of 1 nitrogen atom in ammonia is implied.
In 1 mole of ammonia, we have 1 nitrogen and 3 hydrogen atoms. Since we have 2 moles of ammonia, as indicated in the image above, we can imply that we have 2 nitrogen atoms and 6 hydrogen atoms by multiplying each participating atom of ammonia by 2.
Now that we’ve covered the basics of a compound, let’s move on to determining empirical and molecular formulas.
I. Empirical Formula and Molecular Formula
Chemical compounds form when bonds are formed between atoms. We combine each bonding atom’s element symbol to identify the compound. Here, we have the structural formula of benzene. Benzene is composed of six carbon atoms bonded in a ring. Each carbon in benzene is bonded to a hydrogen atom. For benzene, our molecular formula is C6H6.
The molecular formula shows us the elemental composition of the compound. Looking straight at the molecular formula, we know that we have a compound composed of six carbon atoms and six hydrogen atoms.
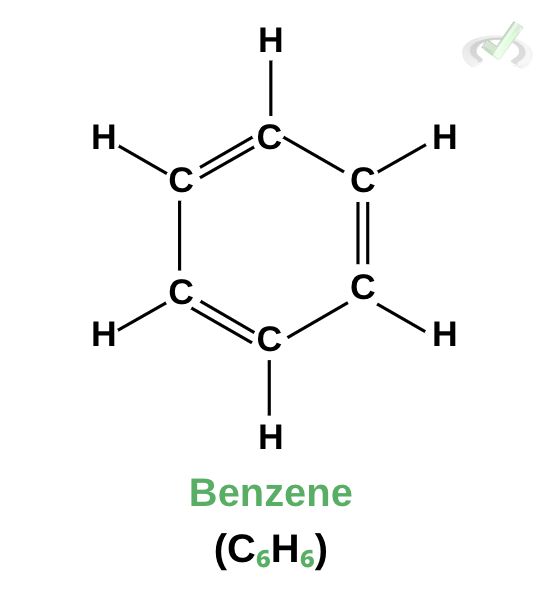
Its empirical formula is CH because this is the compound's ratio of atoms. For each carbon atom, there is 1 hydrogen atom. The empirical formula just tells us the ratio between all the atoms in the compound. The molecular formula, on the other hand, gives us the exact numbers of how many atoms are present in the compound.
Let’s try a few examples of converting a molecular formula to its empirical formula.
Ethane (C₂H₆) has 2 carbon atoms and 6 hydrogen atoms. We can simplify this by dividing each subscript by their common factor, 2. We get an empirical formula of CH3.
C₂H₆ → CH₃
Methane (CH₄) is a compound with 1 carbon and 4 hydrogen atoms. We cannot simplify this further since we already have 1 carbon atom for 4 hydrogen atoms. For an empirical formula, we only want the whole number ratios of each bonding atom. Therefore, the molecular formula of methane is still the same as its empirical formula.
CH₄ → CH₄
Glucose (C₆H₁₂O₆) has carbon, hydrogen, and oxygen. Looking at it directly, we know we can further simplify the formula. We can simplify this by dividing each subscript by their common factor, 2. We then get an empirical formula for glucose, which is CH₂O.
II. Sample Problem
One of the most common word problems in chemistry is identifying the molecular formula given the composition of a compound. Let’s try one problem and work something out.
A scientist found the chemical composition of a compound to be 52.14% carbon, 13.3% hydrogen, and 34.75% oxygen by weight. Determine its molecular formula if its molar mass is 138.204 g/mol.
This problem is quite simple. When given a percent composition problem, we can simply assume that we have 100 units in total. That way, we can effectively relate the numbers without getting the math wrong. This can be done because we’re not changing anything–we’re simply converting the percentage into a more understandable chunk.
A. Assume a 100-g sample of the compound.
If we have 100 grams of the compound, we can say that we have 52.14 grams of carbon, 13.3 grams of hydrogen, and 34.75 grams of oxygen by weight.
B. Convert the mass to moles.
We do this by multiplying the masses by the atomic weight of each individual atom in grams per mole (g/mol).
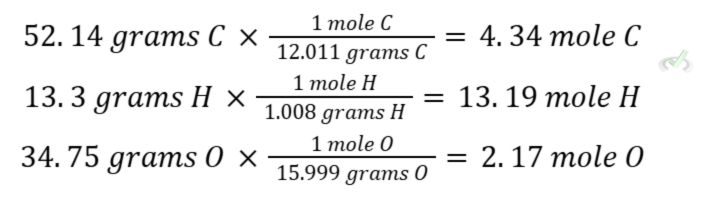
C. Divide the moles by the lowest value.
Among the three, oxygen has the lowest value (2.17 moles). We then proceed to divide each by 2.17.
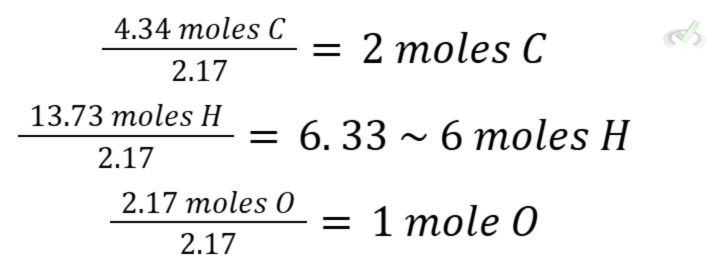
Now, we have the empirical formula C₂H₆O
D. Calculate the molecular formula
We do this by getting the mass of the empirical formula and dividing mass of the compound by the mass of the empirical formula. Lastly, we multiply the subscript of the empirical formula by the quotient we get:
Molar mass of C₂H₆O
(2 x 12.011)+(1.008 x 6)+(1 x 15.999) = 46.069 g/mol
Divide the molar mass of the compound by the mass of the empirical formula.
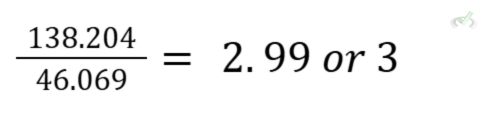
Multiply each subscript by the quotient to get the molecular formula
C₂H₆O → C₂ₓ₃H₆ₓ₃O₁ₓ₃
C₆H1₁₈O₃
III. Conclusion
A compound can have a molecular and an empirical formula. The molecular formula tells us the exact elemental composition of the compound. On the other hand, the empirical formula describes the whole number ratio of atoms in the compound. This is important especially in identifying a compound. Since the empirical formula shows the ratio between bonding atoms, we can get the empirical formula from the molecular formula but we cannot get the molecular formula from the empirical formula.
IV. Key Terms
A compound can have a molecular and an empirical formula. The molecular formula tells us the exact elemental composition of the compound. On the other hand, the empirical formula describes the whole number ratio of atoms in the compound. This is important especially in identifying a compound. Since the empirical formula shows the ratio between bonding atoms, we can get the empirical formula from the molecular formula but we cannot get the molecular formula from the empirical formula.
V. Practice Questions
Nicotine has a molar mass of 162.1 g/mol. A sample of the compound contains 74% carbon, 8.7% hydrogen, and 17.3% hydrogen.
Sample Practice Question 1
Find its empirical formula.
A. C₅H₇N
B. C₁₀H₁₄N₂
C. CHN
D. C₇H₅N
Ans. A
Sample Practice Question 2
Find its molecular formula.
A. C₅H₇N
B. C₁₀H₁₄N₂
C. CHN
D. C₇H₅N
Ans. B







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
