A chemical reaction is a process that involves the transformation of a compound into a new form. In a reaction, the reactant is the one being transformed. Once the reaction takes place, the end product of the reaction is the product.

In a chemical reaction equation, we see that the reactants and products are separated by an arrow.
This arrow will usually point towards the product to indicate the change and the transformation into the final form of the reactant. We cannot go back to reform the product if we’re using an arrow pointing towards one side alone.
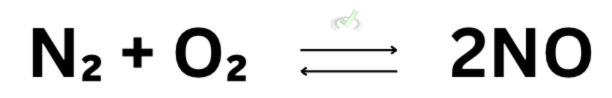
This arrow can also point on both sides, indicating a reversible reaction, meaning the reaction can happen in reverse. This means that the reverse can occur at the same rate or speed. In the reaction of 1 mole of N₂ and O₂ to form 2 moles of NO, the reverse can happen at the same rate or speed. 2 moles of NO can react in reverse and reform its original reactants.
Now that we know the basics of a chemical equation, let’s look at the types of chemical reactions.
I. Synthesis Reaction
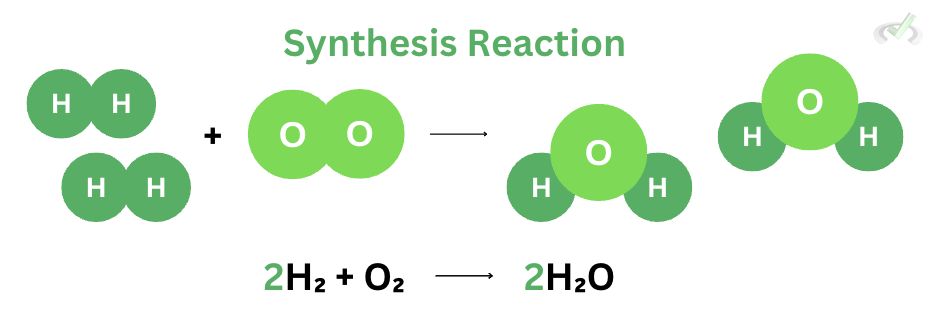
Synthesis reactions are straightforward. They combine two or more reactants to form a single product. Here, we see 2 moles of hydrogen gas (H₂) react with one mole of oxygen gas (O₂) to form 2 moles of water (H₂O). We add reactants to create a single complex product in a synthesis reaction. We use the term synthesis because we’re making or synthesizing something new out of our reactants.
II. Decomposition Reaction
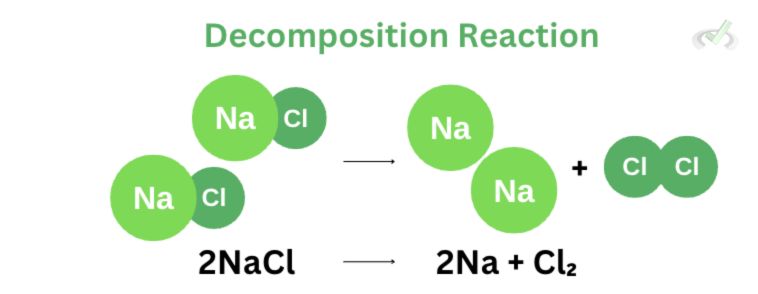
A decomposition reaction is also straightforward. This reaction involves breaking down the reactant into its simpler form. We can think of it as removing ice from coffee. Our reactant would be the iced coffee, and once it undergoes decomposition, we remove all the ice cubes from the coffee and end up with coffee and ice as our product.
We used the decomposition of sodium chloride (NaCl) as an example. NaCl decomposes after undergoing electrolysis (decomposition of ionic compounds) into sodium (Na) and chlorine gas (Cl₂).III. Combustion Reaction
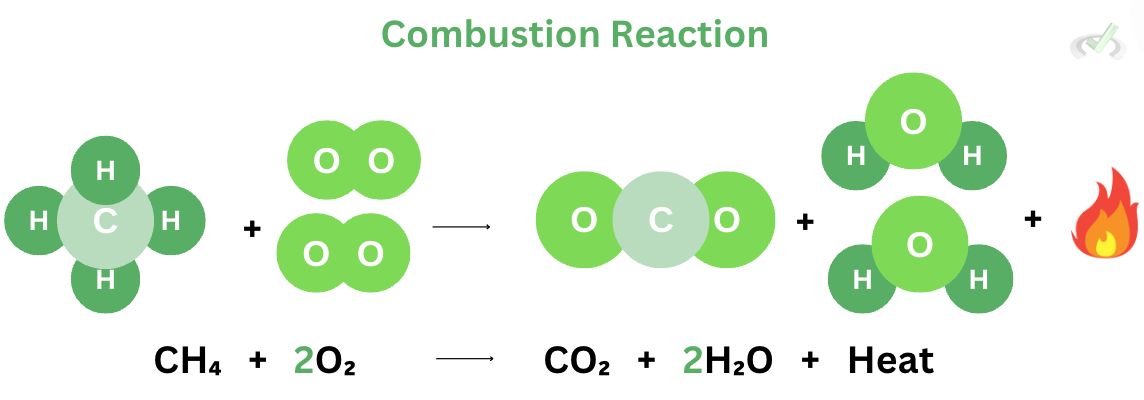
In a combustion reaction, a substance (usually a hydrocarbon) reacts with oxygen to produce heat, carbon dioxide, and water as a byproduct. The gasoline in generators reacts with oxygen to produce energy; we usually see smoke and a bit of water coming out of it, indicating the presence of heat, water, and carbon dioxide.
IV. Single Displacement Reaction
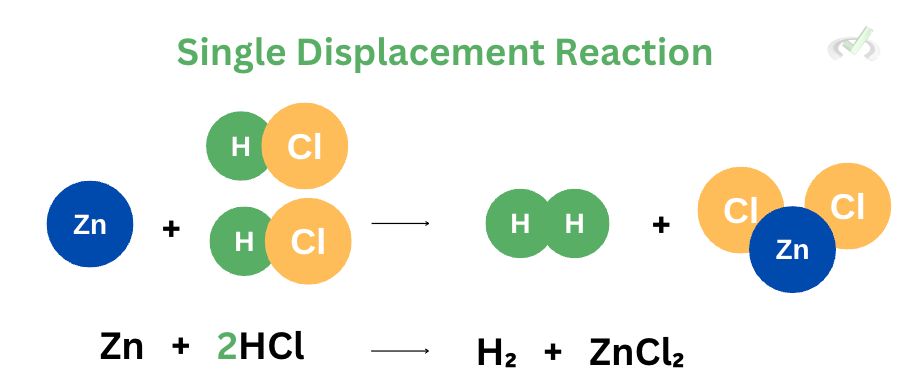
Single displacement reactions involve the displacement of a reactant to form a new product. The example above shows that zinc displaces hydrogen to bond with chlorine. In this process, zinc takes hydrogen’s spot and bonds with chlorine atoms to form zinc chloride. On the other hand, hydrogen gas (H₂) is released to the atmosphere after being ejected.
V. Double Displacement Reaction
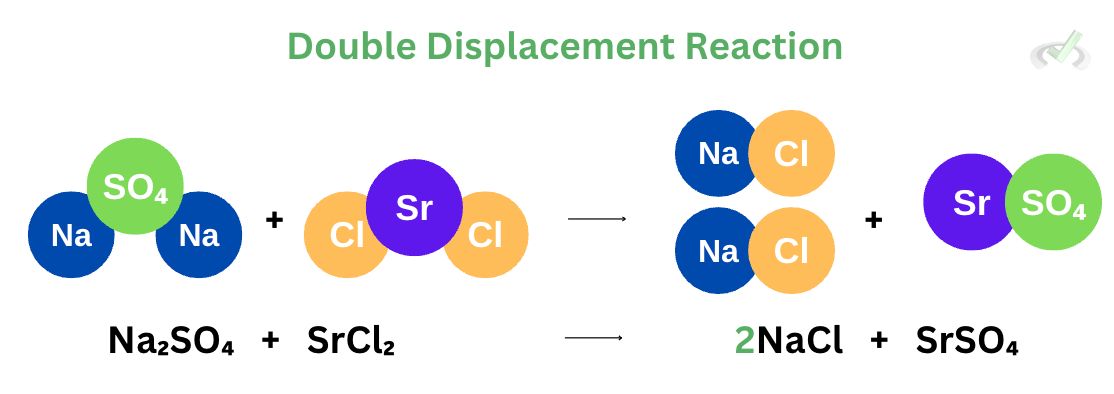
A double displacement reaction involves the exchange of ions in a compound to form a new product. We see in the example above that we have sodium sulfate (Na₂SO₄) and strontium chloride (SrCl₂) react to form 2 moles of sodium chloride (NaCl) and strontium sulfate (SrSO₄). You may notice the exchange of compounds between the two reactants. On the products’ side, sodium is bonded with chlorine, and strontium bonds with sulfate. This is the mechanism of a double displacement reaction–it involves the exchange of compounds or atoms after a reaction.
VI. Conclusion
The mechanism of all the types of reactions can be dumbed down using the image to the right.
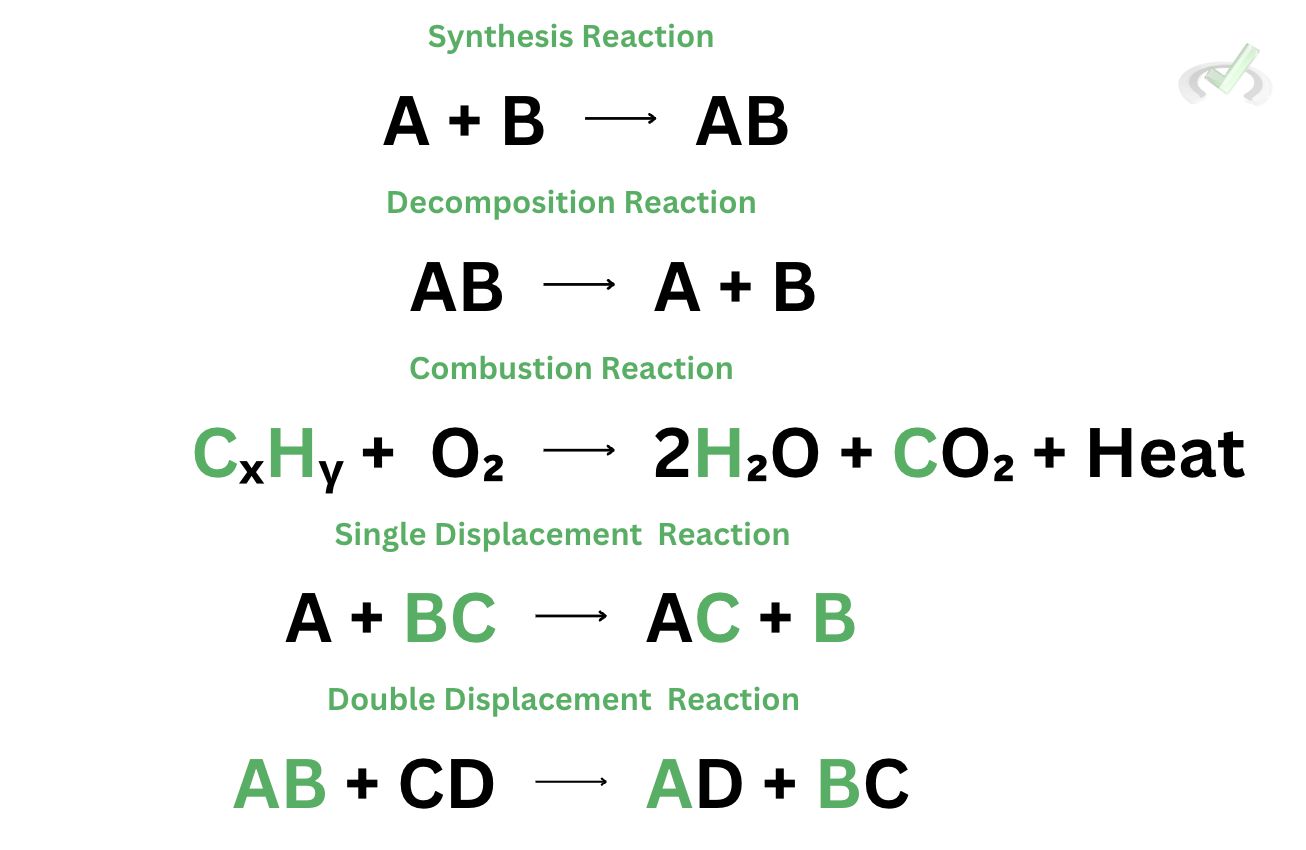
In a synthesis reaction, we’re synthesizing a new product (AB) using a combination of the reactants (A and B). For decomposition reaction, the reactant (AB) decomposes into a much simpler form (A and B). We see heat as a product in a combustion reaction. A hydrocarbon reacts with oxygen to produce heat, water, and carbon dioxide. In a single displacement reaction, we’re displacing a single component (B) of a compound (BC) by replacing it with another reactant (A). Double displacement reactions involve the exchange of ions or elements in a compound. The reactants (AB and CD) exchange bonded atoms or ions (B and D) to form new compounds (AD and BC).
VII. Key Terms
- Chemical Reaction - A reaction that involves the transformation of a reactant into a new compound (product).
- Combustion Reaction - A reaction that involves exposing a substance to oxygen to form heat.
- Decomposition Reaction - A reaction that involves breaking down a reactant into a much simpler form.
- Double Displacement Reaction - A reaction that involves the exchange of ions in compounds to form new compounds.
- Single Displacement Reaction - A reaction that involves replacing an element or an ion in a compound.
- Synthesis Reaction - A reaction that involves combining the reactants to form a new compound.
VIII. Practice Questions
Sample Practice Question 1
Which of the following reactions is not accurate?
A. A + B > AB; Synthesis Reaction
B. AD + BC > AC + BD; Double Displacement Reaction
C. BC + D > BD + C; Single Displacement Reaction
D. AB > C + AB; Decomposition Reaction
Ans. D
Sample Practice Question 2
Which of the following statements is incorrect?
A. One of the products of a combustion reaction is energy.
B. A chemical reaction involves changes in a reactant.
C. A product can revert into a reactant if the reaction is in equilibrium.
D. A chemical reaction needs a catalyst to take place.
Ans. D







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
