Gases are an essential part of chemistry. They follow specific laws and have unique properties. Understanding these can help in many scientific fields. Here, we’ll go through important gas-related terms and variables, with clear explanations and examples.
I. Introduction to Gases
Gases are one of the four states of matter, alongside solids, liquids, and plasmas. They have unique properties that distinguish them from the other states. Let's explore some basic concepts:
A. Properties of Gases
- Compressibility: Gases can be compressed because their molecules are spread out. For example, when you pump air into a bicycle tire, the gas molecules are pushed closer together.
- Expansibility: Gases expand to fill any container they're in. This is why a helium balloon will expand as you add more helium.
- Low Density: Gases have much lower density compared to solids and liquids. This is why a balloon filled with air is much lighter than the same balloon filled with water.
B. Important Variables
- Pressure (P): The force exerted by molecules of gas when they collide with the walls of their container. Measured in units like atmospheres (atm), pascals (Pa), or millimeters of mercury (mmHg). For example, tire pressure is often measured in psi (pounds per square inch), another unit of pressure.
- Volume (V): The space that gas occupies. Measured in liters (L) or cubic meters (m³). An example is the air volume in a balloon, which increases as you blow more air into it.
- Temperature (T): The measure of the average kinetic energy of gas molecules. Measured in Kelvin (K). For instance, heating a gas will increase its temperature and cause the molecules to move faster.
- Number of Moles (n): The amount of gas measured in moles (mol). One mole is 6.022 x 10²³ molecules. This is a large number, but it helps scientists measurably count particles.
II. Gas Laws
Gas laws describe the relationship between these variables. Here are the most important ones:
A. Boyle’s Law
Boyle’s Law states that the pressure of a gas is inversely proportional to its volume when temperature and the number of moles are constant. Mathematically:
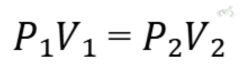
Example: If the volume of a gas decreases, its pressure increases, provided the temperature remains constant. Imagine a syringe filled with air. If you push the plunger in, the volume decreases, and the pressure increases.
B. Charles’s Law
Charles’s Law states that the volume of a gas is directly proportional to its temperature (in Kelvin) when pressure and the number of moles are constant. Mathematically:
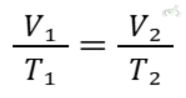
Example: Heating a balloon causes it to expand because the gas volume increases with temperature. This is why a hot air balloon rises. The air inside the balloon is heated, causing it to expand and become less dense than the cooler air outside.
C. Avogadro’s Law
Avogadro’s Law states that the volume of a gas is directly proportional to the number of moles of gas when pressure and temperature are constant. Mathematically:
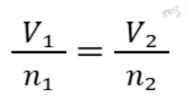
Example: Adding more gas molecules into a container increases its volume if temperature and pressure stay constant. For example, blowing more air into a balloon increases its size.
D. Ideal Gas Law
The Ideal Gas Law combines Boyle’s, Charles’s, and Avogadro’s laws into one equation:
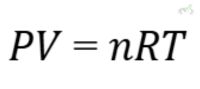
Example: This law helps calculate the behavior of an ideal gas under various conditions of pressure, volume, and temperature. If you know the amount of gas (in moles), the temperature, and the volume, you can calculate the pressure.
E. Dalton’s Law of Partial Pressures
Dalton’s Law states that the total pressure of a mixture of gases is the sum of the partial pressures of individual gases. Mathematically:
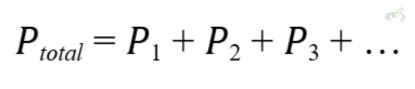
Example: In a mixture of oxygen and nitrogen, the total pressure is the sum of the pressures exerted by each gas independently. This is why the pressure in a scuba tank, which contains a mixture of oxygen and nitrogen, is the sum of the pressures of both gases.
III. Important Concepts and Equations
A. Molar Volume
At standard temperature and pressure (STP: 0°C and 1 atm), one mole of an ideal gas occupies 22.4 liters.
B. Gas Stoichiometry
In chemical reactions involving gases, we use the ideal gas law to relate the amounts of reactants and products.
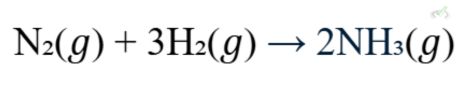
Using the ideal gas law, you can determine how much NH₃ will be produced from a given amount of N₂ and H₂.
C. Kinetic Molecular Theory
This theory explains the behavior of gases, stating that gas particles are in constant, random motion and that their collisions are perfectly elastic.
D. Real Gases
Real gases deviate from ideal behavior at high pressures and low temperatures. The Van der Waals equation modifies the ideal gas law to account for these deviations:
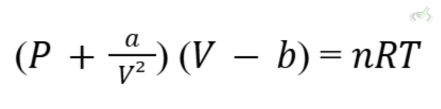
- a: Corrects for intermolecular forces.
- b: Corrects for the volume occupied by gas molecules.
IV. Applications and Examples
A. Airbags
Airbags in cars use rapid gas production to inflate in case of a collision.
B. Breathing
Human breathing relies on exchanging oxygen and carbon dioxide gases in the lungs. When we inhale, our lungs expand, decreasing the pressure inside and drawing in air. When we exhale, our lungs contract, increasing the pressure and pushing air out.
C. Industrial Processes
Ammonia production through the Haber process is a vital industrial reaction involving gases.
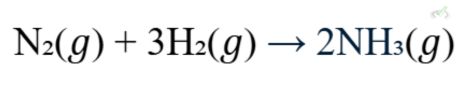
V. Bridge/Overlap
A. Thermodynamics
Gas laws are essential in understanding energy changes in chemical reactions. For example, the expansion or compression of gases can be related to work and heat, which are key concepts in thermodynamics.
B. Chemical Equilibrium
Understanding gas behavior is crucial for studying reactions in closed systems. For example, Le Chatelier's Principle states that if a system at equilibrium is disturbed, it will adjust to minimize the disturbance. This principle helps predict how changing pressure, volume, or temperature will affect gas reactions at equilibrium.
C. Environmental Chemistry
Gas behavior and reactions are significant in atmospheric chemistry and pollution control. For example, the formation and breakdown of ozone in the atmosphere involve gas-phase reactions crucial for protecting every life on Earth from harmful UV radiation.
VI. Wrap-Up and Key Terms
Key Terms
- Pressure (P): Force per unit area exerted by gas molecules.
- Volume (V): Space occupied by gas.
- Temperature (T): Measure of average kinetic energy of gas molecules.
- Number of Moles (n): Amount of gas.
Key Equations
- Boyle’s Law:
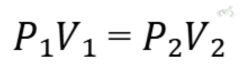
- Charles’s Law:
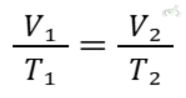
- Avogadro’s Law:
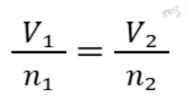
- Ideal Gas Law:
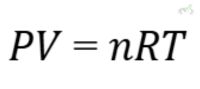
- Van der Waals Equation:
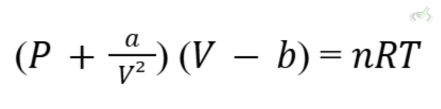
VII. Practice Questions
Sample Practice Question 1
What happens to the pressure of a gas if its volume is halved, assuming the temperature remains constant?
A. It doubles.
B. It halves.
C. It stays the same.
D. It quadruples.
Ans. A
According to Boyle’s Law, if the volume decreases, pressure increases.
Sample Practice Question 2
What is the volume of one mole of an ideal gas at STP?
A. 1 liter.
B. 22.4 liters.
C. 100 liters.
D. 0.082 liters.
Ans. B
At standard temperature and pressure, one mole of an ideal gas occupies 22.4 liters.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
