The Kinetic Molecular Theory (KMT) explains the behavior of gases. It states that gas particles are in constant, random motion. This theory helps us understand gas properties like pressure, temperature, and volume.
I. Introduction to Kinetic Molecular Theory
Kinetic Molecular Theory describes the behavior of gas particles. It is based on several key assumptions. Let's explore these assumptions in detail.
Assumption 1: Particle Motion
Gas particles are in constant, random motion. They move in straight lines until they collide with each other or the walls of their container. These collisions are elastic, meaning no energy is lost. This means that the total kinetic energy of the gas particles remains the same after collisions.
Assumption 2: Negligible Particle Volume
The volume of individual gas particles is very small compared to the volume of the container. This means we can ignore the particles' volume.
Assumption 3: No Attractive Forces
There are no forces of attraction or repulsion between gas particles. They do not stick to each other or push each other away, so they do not influence each other's motion.
II. Gas Laws and Kinetic Molecular Theory
KMT helps explain the gas laws, which describe how gases behave under different temperature, pressure, and volume conditions. Gas laws are mathematical relationships that describe how gases respond to environmental changes.
A. Boyle's Law (Pressure and Volume)
Boyle's Law states that the pressure of a gas is inversely proportional to its volume when temperature is constant.
Equation:
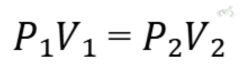
Example: If you compress a gas (reduce its volume), its pressure increases. If you expand the gas (increase its volume), its pressure decreases.
B. Charles's Law (Temperature and Volume)
Charles's Law states that the volume of a gas is directly proportional to its temperature when pressure is constant.
Equation:
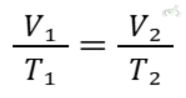
Example: If you heat a gas, it expands. If you cool a gas, it contracts.
III. Real Gases vs. Ideal Gases
KMT assumes gases are ideal, meaning they follow the gas laws perfectly. However, real gases can deviate from ideal behavior, especially under high pressure or low temperature.
Deviations from Ideal Behavior
Gas particles are forced closer together at high pressure, so their volume is no longer negligible. At low temperatures, gas particles move more slowly, and attractive forces become more significant.
Van der Waals Equation:
This equation adjusts the ideal gas law to account for these deviations:
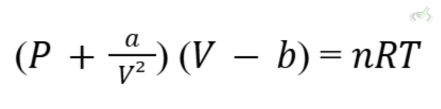
- a: accounts for attractive forces.
- b: accounts for the volume of gas particles.
IV. Applications of KinUsing the Van der Waals equation, atic Molecular Theory
Understanding KMT helps explain manations.
A. Diffusion
Diffusion is the process of gas particles spreading out to evenly fill a container. This happens because of the random motion of the particles.
Example: When you spray perfume in one corner of a room, it eventually spreads throughout the room due to the random motion of gas particles.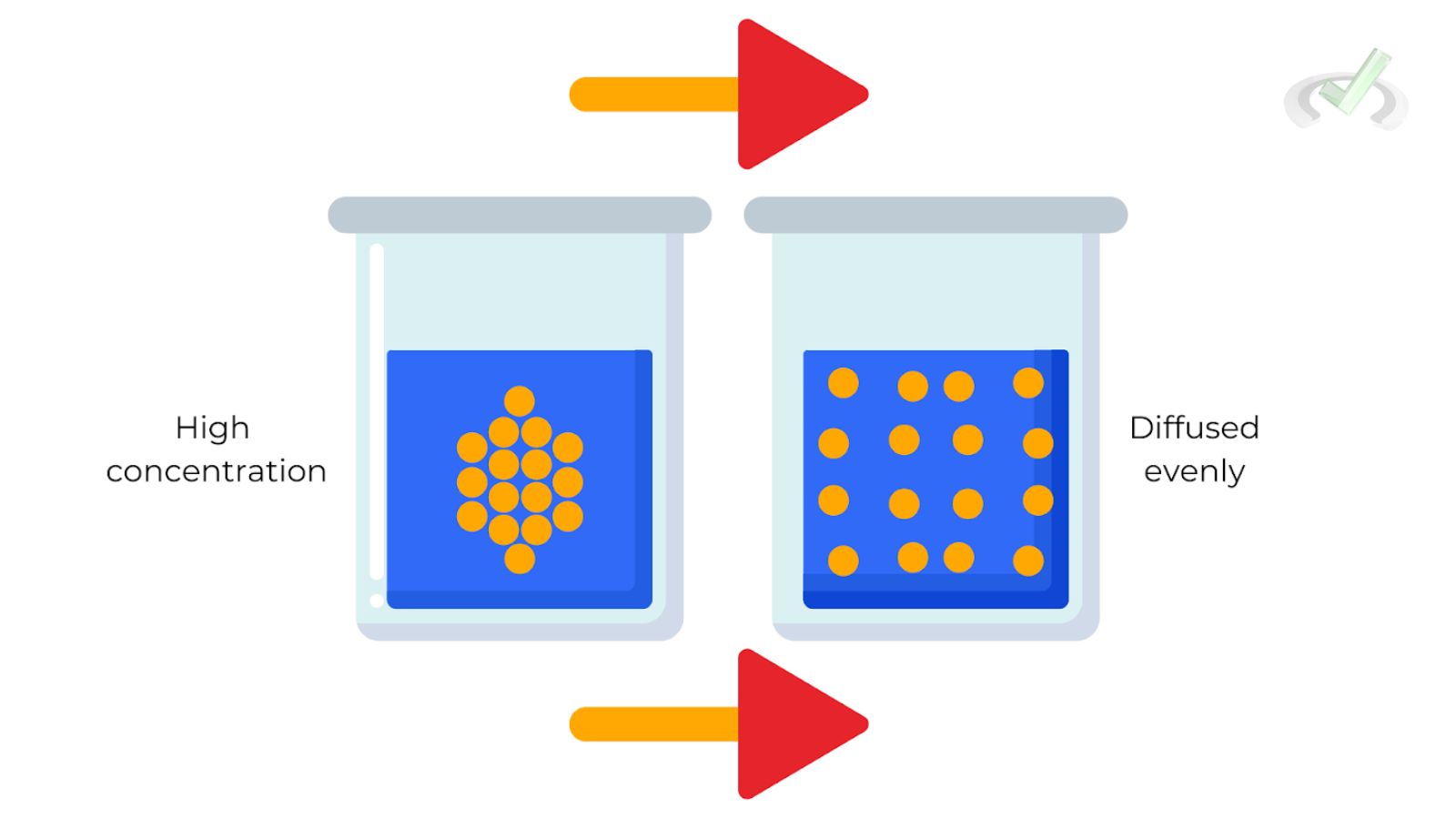
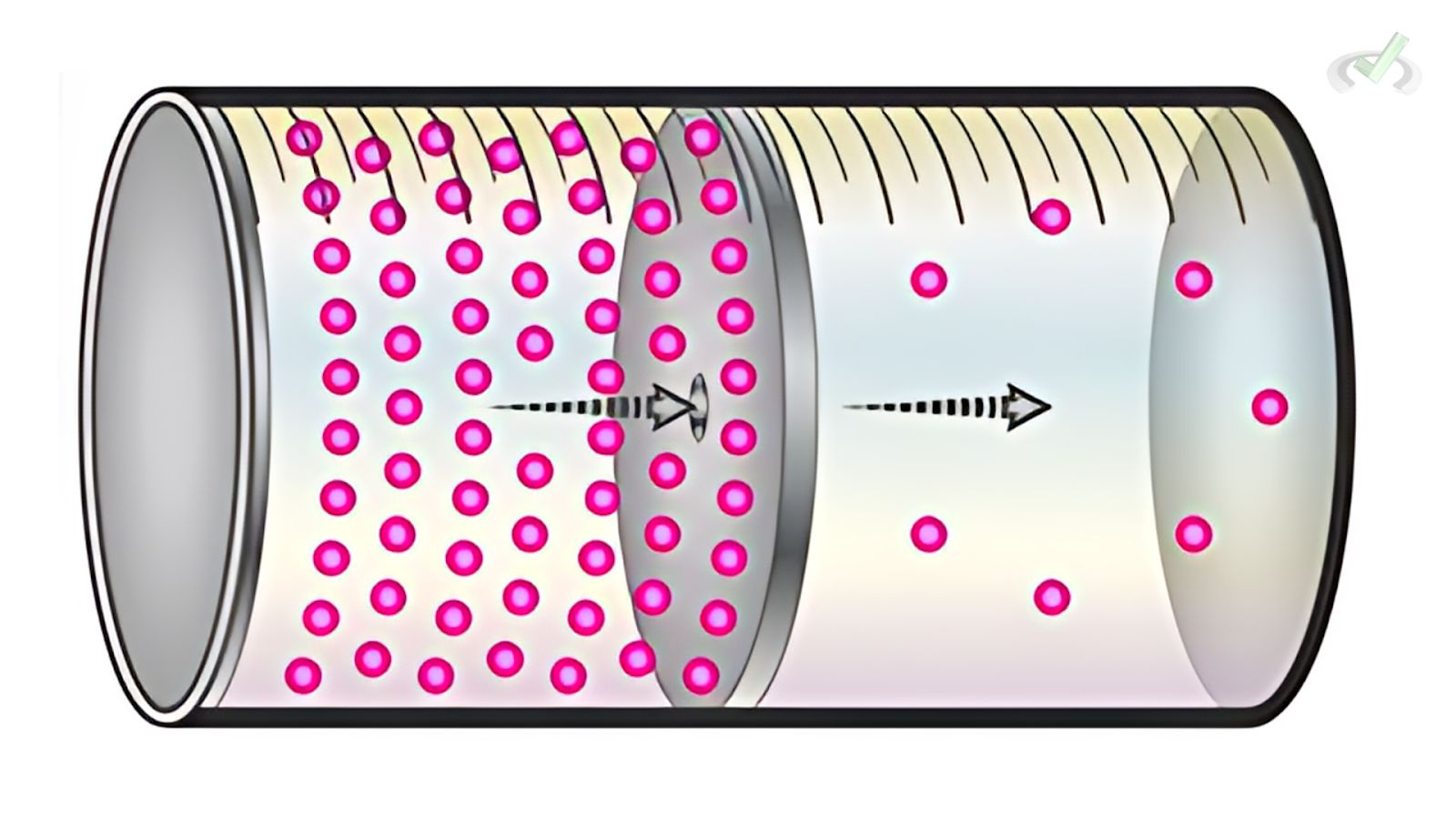
B. Effusion
Effusion is the process of gas particles passing through a small opening. According to Graham's Law, the effusion rate is inversely proportional to the square root of the gas's molar mass.
Equation:
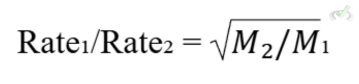
Example: Lighter gases (like hydrogen) effuse faster than heavier gases (like oxygen).
V. Bridge/Overlap
A. Temperature and Kinetic Energy
Temperature is a measure of the average kinetic energy of gas particles. Higher temperature means higher kinetic energy and faster-moving particles.
Equation:
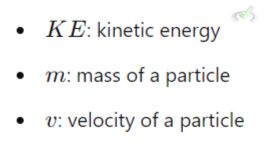
B. Heat and Work
In thermodynamics, heat transfers energy due to a temperature difference, and work transfers energy when a force moves an object. KMT helps explain how gas particles transfer energy as heat or work.
VI. Wrap-Up and Key Terms
Let's review some key terms and concepts from Kinetic Molecular Theory.
- Elastic Collisions: Collisions where no energy is lost.
- Boyle's Law: P1V1=P2V2
- Charles's Law: V1/T1=V2/T2
- Ideal Gas: A gas that follows gas laws perfectly.
- Real Gas: A gas that deviates from ideal behavior under certain conditions.
- Diffusion: Spreading of gas particles throughout a container.
- Effusion: Passing of gas particles through a small opening.
VII. Practice Questions
Sample Practice Question 1
What happens to the pressure of a gas if its volume is halved while temperature remains constant?
A. It doubles
B. It halves
C. It stays the same
D. It quadruples
Ans. A
According to Boyle's Law, pressure and volume are inversely proportional. Halving the volume doubles the pressure.
Sample Practice Question 2
How does the volume of a gas change if its temperature is increased while pressure remains constant?
A. It increases
B. It decreases
C. It stays the same
D. It becomes zero
Ans. A
According to Charles's Law, volume and temperature are directly proportional. Increasing the temperature increases the volume.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
