Thermodynamics is the study of heat, energy, and work. In chemistry, it helps us understand how energy changes during chemical reactions. This guide covers key terms, processes, and functions in thermodynamics.
I. Introduction to Thermodynamics
Thermodynamics explains how heat, energy, and work move and change in chemical reactions. Here are some basic terms:
A. System and Surroundings
A system is the part of the universe we are studying. Everything outside the system is the surroundings. For example, in a reaction inside a beaker, the system is the chemicals in the beaker. The surroundings are the beaker and the air around it.
B. Types of Systems
There are three types of systems:
- Open System: Can exchange both energy and matter with its surroundings. Example: A boiling pot of water.
- Closed System: It can exchange energy but does not matter with its surroundings. Example: A sealed flask.
- Isolated System: Cannot exchange energy or matter with its surroundings. Example: A thermos flask.
C. State Functions
State functions depend only on the state of the system, not how it got there. Examples include temperature, pressure, and volume. These are important because they help us understand the energy changes in reactions.
II. Key Thermodynamic Terms and Equations
Understanding these terms and equations helps in studying thermodynamics:
A. Internal Energy (U)
Internal energy is the total energy within a system. It includes all kinetic (motion) and potential (stored) energy of the particles. For example, heating water in a pot increases its internal energy.
ΔU=q+w
where q is heat added to the system, and w is work done on the system.
B. Enthalpy (H)
Enthalpy is the heat content of a system at constant pressure. When water boils, it absorbs heat, changing its enthalpy.
ΔH=ΔU+PΔV
where P is pressure, and ΔV is the change in volume.
C. Entropy (S)
Entropy measures the disorder or randomness in a system. Higher entropy means more disorder. For example, when ice melts into water, the entropy increases because the water molecules move more freely.
Where qᵣₑᵥ is the reversible heat, and T is temperature in Kelvin.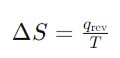
D. Gibbs Free Energy (G)
Gibbs Free Energy determines if a reaction is spontaneous (happens on its own). A negative ΔG means the reaction is spontaneous. For example, the rusting of iron is spontaneous because ΔG is negative.
ΔG=ΔH−TΔS
III. Thermodynamic Processes
Different processes affect the system in various ways:
A. Isothermal Process
An isothermal process occurs at a constant temperature. In this process, the internal energy change (ΔU) is zero.
B. Adiabatic Process
An adiabatic process occurs without heat exchange. All energy changes come from work done on or by the system.
C. Isochoric Process
An isochoric process occurs at constant volume. No work is done because the volume does not change. Any heat added changes the internal energy.
D. Isobaric Process
An isobaric process occurs at constant pressure. The heat added or removed changes the enthalpy.
IV. Important Thermodynamic Functions
These functions are key to understanding how energy changes:
A. Heat Capacity (C)
Heat capacity is the amount of heat needed to change the temperature of a substance by 1 degree Celsius. It is defined as:
Where q is heat added, and ΔT is the change in temperature.
B. Standard Enthalpy of Formation (ΔHf°)
The standard enthalpy of formation is the heat change when one mole of a compound is formed from its elements in their standard states.
C. Standard Entropy (S°)
Standard entropy is the absolute entropy of a substance at 1 atm pressure and 298 K.
D. Standard Gibbs Free Energy Change (ΔG°)
The standard Gibbs Free Energy change is the change in free energy for a reaction under standard conditions (1 atm, 298 K).
V. Chemical Equations and Examples
Including equations helps illustrate thermodynamic concepts:
A. Combustion of Methane
The combustion of methane (CH₄) is an exothermic reaction:
CH₄+2O₂→CO₂+2H₂O
ΔH for this reaction is negative, indicating it releases heat. This is why methane is used as fuel.
B. Formation of Water
The formation of water from hydrogen and oxygen is another example:
2H₂+O₂→2H₂O₂
ΔH is also negative. This reaction is important in respiration and combustion.
C. Dissolution of Sodium Chloride
The dissolution of sodium chloride (NaCl) in water is an endothermic process:
NaCl(s)→Na+(aq)+Cl−(aq)
ΔH is positive, meaning it absorbs heat from the surroundings.
VI. Bridge/Overlap
Thermodynamics connects with many other areas in chemistry:
A. Chemical Kinetics
Understanding energy changes helps explain reaction rates. Higher temperatures often increase reaction rates by providing more energy. This explains why food cooks faster at higher temperatures.
B. Physical Chemistry
Thermodynamics is crucial in physical chemistry, explaining how energy changes in different states of matter. For example, it helps understand phase transitions like melting and boiling.
C. Environmental Chemistry
Thermodynamic principles help explain how pollutants behave in the environment. For example, they predict how gases will dissolve in oceans, affecting marine life and the atmosphere.
D. Biochemistry
In biochemistry, thermodynamics helps us understand metabolic pathways. For example, the breakdown of glucose in cellular respiration releases energy that cells use to perform work.
VII. Wrap-Up and Key Terms
Understanding thermodynamics involves mastering several key terms and concepts. Let's review:
Key Terms
- System: Part of the universe being studied.
- Surroundings: Everything outside the system.
- Internal Energy (U): Total energy within a system.
- Enthalpy (H): Heat content at constant pressure.
- Entropy (S): Measure of disorder.
- Gibbs Free Energy (G): Determines if a reaction is spontaneous.
VIII. Practice Questions
Sample Practice Question 1
What is the change in internal energy (ΔU) if a system absorbs 50 J of heat and does 20 J of work on the surroundings?
A. 30 J
B. 70 J
C. -30 J
D. -70 J
Ans. A
ΔU = q + w. Here, q = 50 J (heat absorbed) and w = -20 J (work done by the system). So, ΔU = 50 J + (-20 J) = 30 J.
Sample Practice Question 2
In which type of process does the volume of the system remain constant?
A. Isothermal
B. Adiabatic
C. Isochoric
D. Isobaric
Ans. C
An isochoric process occurs at constant volume, meaning no work is done because the volume does not change.







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
