Aldehydes and ketones, just like other organic molecules, are common in our daily lives! When you all get accepted into medical school and start doing cadaver labs, you’ll likely encounter the strong pungent odor of formaldehyde! You may have also heard about ketones, for better or worse, and how their production increases when on the keto diet!
This section will be particularly important because it's our first introduction and encounter of the carbonyl which is, without doubt, one of the most important functional groups in organic chemistry!
After this chapter overview, you’ll have a brief understanding of the basic characteristics and reactions of aldehydes and ketones. Let’s get started!
Aldehydes and Ketones on the MCAT: What You Need to Know
Topics on organic chemistry will be tested on the Chem/Phys and the Bio/Biochem section of the MCAT and can appear both as passage based and fundamental discrete questions.
This section is similar to the one covering carboxylic acids and its derivatives, as it’s also a heavily tested MCAT organic chemistry topic. We calculated around a 6-8 question average that you can probably expect.
Introductory organic chemistry accounts for 15% of the content covered in the Chemical and Physical Foundations of Biological Systems and 5% of the content covered in the Biological and Biochemical Foundations of Living Systems.
Important Sub-Topics: Aldehydes and Ketones
As mentioned above, the section is quite crucial due to the introduction and importance of the carbonyl functional group. You’ll probably notice some similarities in the reactions, specifically the mechanism) between aldehydes/ketones and carboxylic acids as covered in our other chapter overview!
1. Basic Structure and Nomenclature of Aldehydes and Ketones
The structures of aldehydes and ketones are connected by one commonality: they both contain a carbonyl group, which is composed of a central carbon double bonded to an oxygen atom.
They only differ in the other atoms attached to the central (also called carbonyl carbon). While both are attached to a carbon molecule, designated by the R, the carbonyl carbon of aldehydes are bonded to a hydrogen atom while ketones are bonded to another carbon based molecule, designated by another R’ as shown below!
Just like when naming other organic molecules, you must first assess if the aldehyde or ketone is the highest priority functional group! Remember, when comparing the 2, aldehydes have higher priority than ketones!
If the aldehyde is the higher priority functional group, replace the “-ane” from its corresponding alkane with the suffix “-anal”. If it’s not the highest priority functional group, we use the prefix “-oxo” just like we use “-hydroxy” for alcohol.
Note that because the aldehyde’s carbonyl is connected to a hydrogen atom, it will always be located at the end of the carbon chain! This is just a small thing to keep in mind when numbering the carbon.
If the ketone is the highest priority functional group, replace the “-e” from the corresponding alkane with the suffix “-one.” If it’s not the highest priority, the prefix “-oxo” or “-keto” can be used!
We can differentiate between if the substituent is an aldehyde or ketone based on the number of carbons and the numbering. Because the aldehyde is at the end, it should always be located on the last number carbon. For example, as shown above, the 4-oxobutanoic acid (4 carbons) has an aldehyde located on the end!

Notice how because 4-oxopentanoic acid is a 5-carbon molecule, the “-oxo” prefix indicates that a ketone is present instead of an aldehyde. If it was an aldehyde, it should be named 5-oxopentanoic acid so that the aldehyde is located at the end.
Full Study Notes : Basic characteristics and nomenclature of aldehydes and ketones
For more in-depth content review on basic characteristics and nomenclature of aldehydes and ketones, check out these detailed lesson notes created by top MCAT scorers.
2. Nucleophilic Addition Reaction of Aldehydes and Ketones
The carbonyl group present in aldehydes, ketones and other molecules is so important because the dipole generated via the differing electronegativity between carbon and oxygen gives the carbon partial positive charge and electrophilic character.
A common motif seen in most organic chemical reactions of aldehydes and ketones is that a nucleophile will attack the electrophilic carbonyl carbon. This results in the movement of a pair of electrons from the double bond to the oxygen atom. The oxygen atom will then be protonated in order to stabilize its negative charge.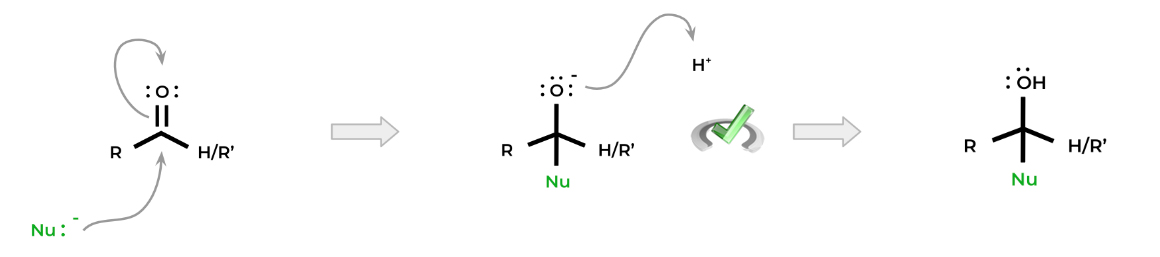
Notice how in the title, it says nucleophilic addition reactions instead of nucleophilic substitution reactions. This is because none of the groups attached to the carbonyl carbon are good leaving groups!
In most cases, the nucleophile will be added to the molecule forming a tetrahedral structure. Another way of thinking about this is that the carbonyl double bond cannot be reformed.
Let’s get introduced to a couple of common reactions involving aldehydes and ketones below! We’ll go into more depth on the superficial reaction mechanism but just keep in mind the generalized mechanism shown above!
Hydration is a common reaction for aldehydes and ketones and is exactly what it sounds like! We can add water with some catalytic acid or base in order to general geminal diols, which are alcohols with 2 hydroxyl groups on the same carbon!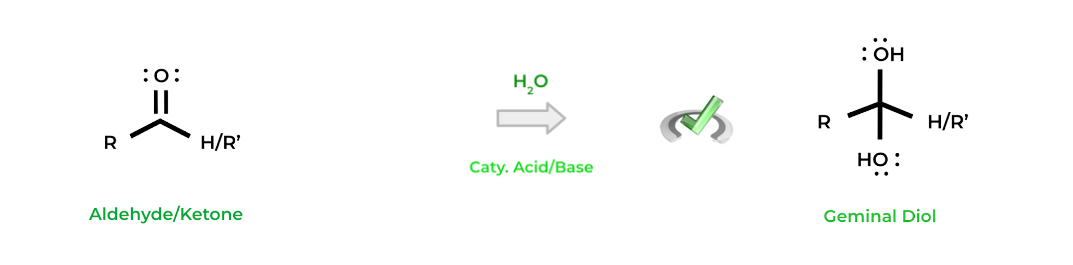
Another common reaction is acetal and ketal formation (and the hemiacetal and hemiketal intermediate). Acetals and ketals are similar to geminal diols except the diols are replaced by alkoxide groups. Recall that these substituents are ethers!
For the hemiacetal/ketal intermediates, only one equivalent of alcohol is needed and can be done under any conditions (acid, basic, or neutral). An additional equivalent of alcohol is needed to complete the reaction to general the acetal and ketal and must be under acidic conditions.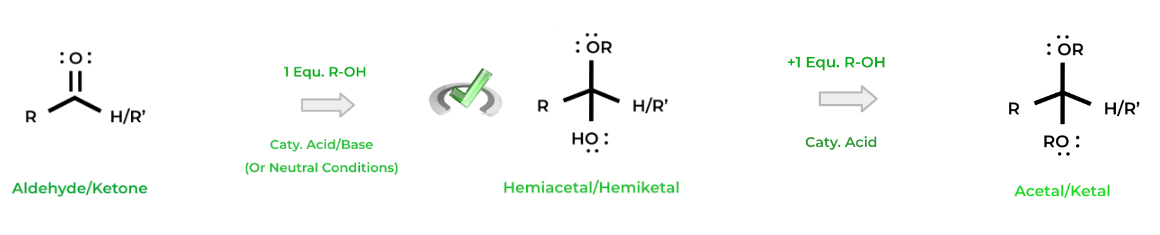
You may have already been introduced to these organic molecules if you’ve covered our “Alcohols and Ethers” chapter overview, as cyclic acetals and ketals could be utilized as protecting groups!
Finally, another key reaction is the formation of cyanohydrins from aldehydes and ketones, which is basically when a cyanide ion (CN-) acts in a nucleophilic addition reaction. Usually, a cyanide salt is utilized along with acidic conditions to catalyze the reaction!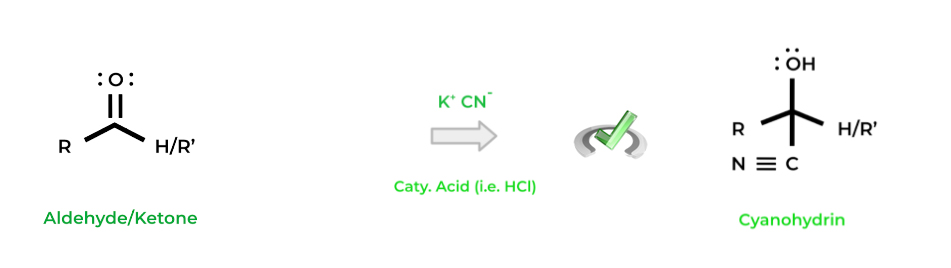
Notice how here we’ve created a new carbon-carbon bond and have also added a carbon-nitrogen bond. These characteristics make cyanohydrins great starting points for synthesizing carboxylic and amino acids.
Full Study Notes : Nucleophilic Addition Reaction of Aldehydes and Ketones
For more in-depth content review on Nucleophilic Addition Reaction of Aldehydes and Ketones, check out these detailed lesson notes created by top MCAT scorers.
3. Redox Reactions of Aldehydes and Ketones
Similar to when we covered the redox reactions of alcohols in our other chapter overview, we’ll again emphasize the general principles of oxidation and reduction in the context of organic chemistry.
Oxidation generally refers to increasing the number of bonds towards oxygen (or more electronegative atoms than carbon) while reducing the number of bonds to hydrogen. Conversely, reduction involves increasing the number of bonds toward hydrogen and decreasing the number of bonds towards oxygen.
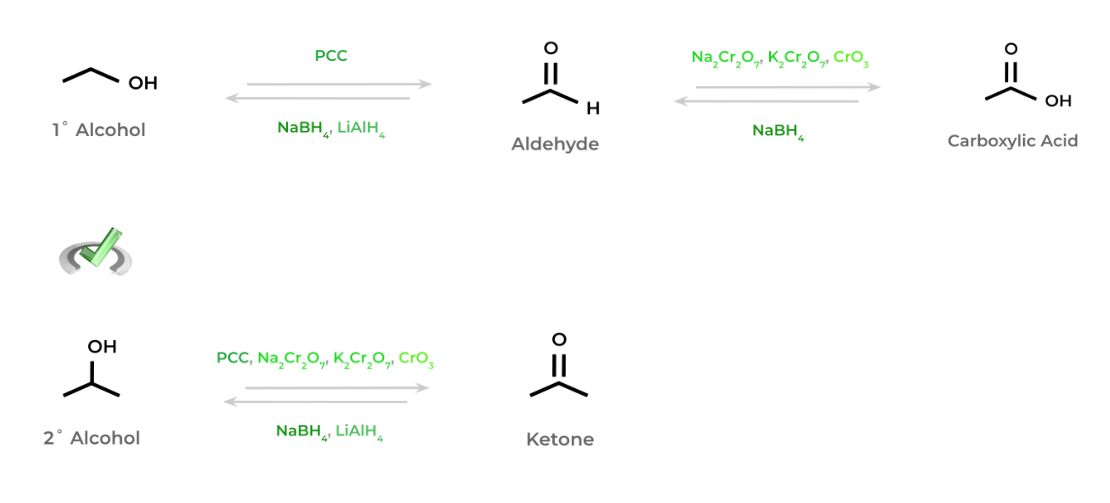
Similar to our reasoning for tertiary alcohols, ketones can’t be further oxidized to carboxylic acids because it requires the breaking of a carbon-carbon bond which is highly unfavorable.
Compare this to the aldehyde, which can be oxidized to a carboxylic acid as it only requires the breaking of a carbon-hydrogen bond. Compared to the breaking of a carbon-carbon bond, this is much more favorable.Full Study Notes : Common redox reactions of aldehydes and ketones
For more in-depth content review on common redox reactions of aldehydes and ketones, check out these detailed lesson notes created by top MCAT scorers.
4. Keto-Enol Tautomerism
Though it might be a scary and daunting set of words, it’s actually quite simple when put side by side! Before getting into what the term “keto-enol” tautomerism means as a whole, we first have to discuss a little more about the formation of enolates.
Enolates are formed when a hydrogen on the alpha carbon is deprotonated via a strong base. The alpha carbon is the next adjacent carbon to the carbonyl carbon – note that because of this, ketones have 2 alpha carbons.
This acidity comes from the electron-withdrawing nature of the oxygen carbonyl atom. When it deprotonates the hydrogen, the electron pair moves back onto the carbon atom, forming an enolate ion.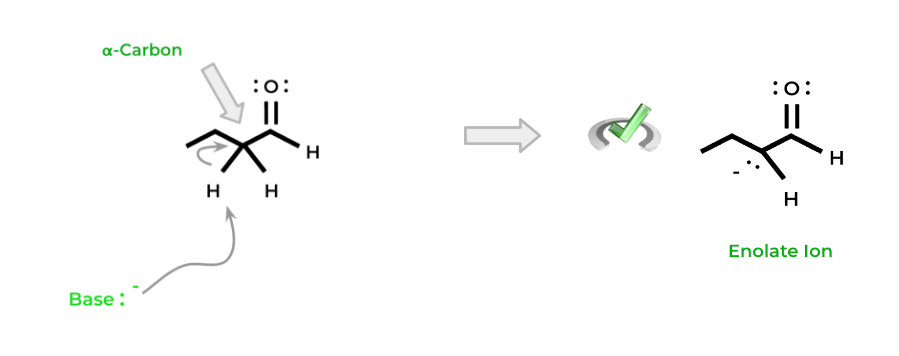
To increase the stability of the enolate ion, we can move the electron pair to form a carbon-carbon double bond, as shown below. This results in the pushing of an electron pair from the carbonyl double bond onto the oxygen – this configuration is more stable as it places the electron pair on the more electronegative oxygen.
Finally, to form the enol, the added electron pair on the oxygen atom can be protonated, which results in an alcohol. Now the name should make sense — the “-en” portion comes from the fact that an alkene is formed via the carbon-carbon double bond. The “-ol” portion means that there's an alcohol group attached!
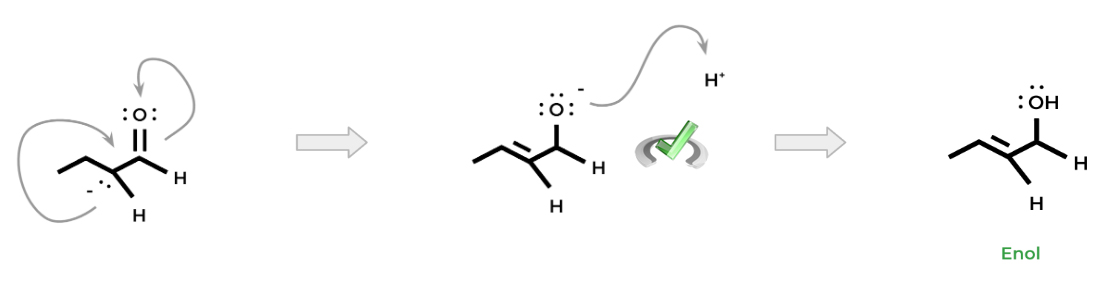
Under these conditions, where it’s possible to be attacked by a strong base, there will be an equilibrium between the “keto” and “enol” forms. The “keto” form is simply the aldehyde or ketone, while the “enol” form refers to the enol (duhh…).
In most cases, when placed in equilibrium with one another, the “keto” form is favored because of the greater strength of the carbon-oxygen double bond compared to the carbon-carbon double bond of the “enol” form.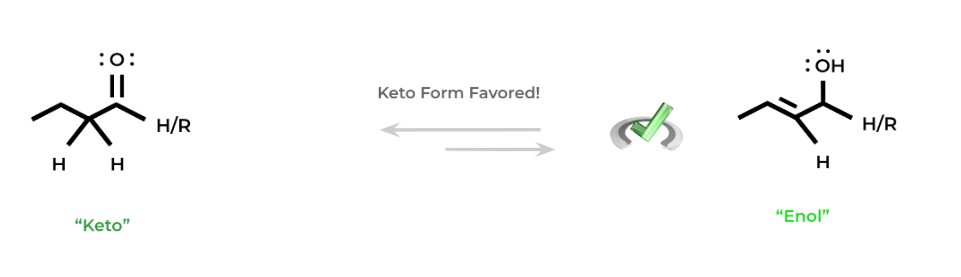
Another important note that will touch upon more in our article is that when performing the deprotonation of an 𝜶-hydrogen to form an enolate, you must use a strong sterically hindered base, such as tert-butoxide!
This is because strong bases can also act as a nucleophile and attack the carbonyl carbon directly! If we use a strong, sterically hindered base, we reduce the possibility of targeting the carbonyl carbon because sterically hindered bases are unfavorable for nucleophilic attack!Full Study Notes : Enolate chemistry and keto-enol tautomerization of aldehydes and ketones
For more in-depth content review on enolate chemistry and keto-enol tautomerization of aldehydes and ketones, check out these detailed lesson notes created by top MCAT scorers.
5. Aldol Addition and Condensation
One important characteristic of enolates is that they can actually function fairly well as nucleophiles! For simplicity purposes, this is because of the carbanion that’s formed due to deprotonation of the 𝜶-hydrogen.
Additionally, recall that in the presence of a strong base, aldehydes and ketones can form enolate ions. These enolate ions can actually act as nucleophiles and attack the carbonyls of the aldehydes and ketones remaining in solution in their keto form!
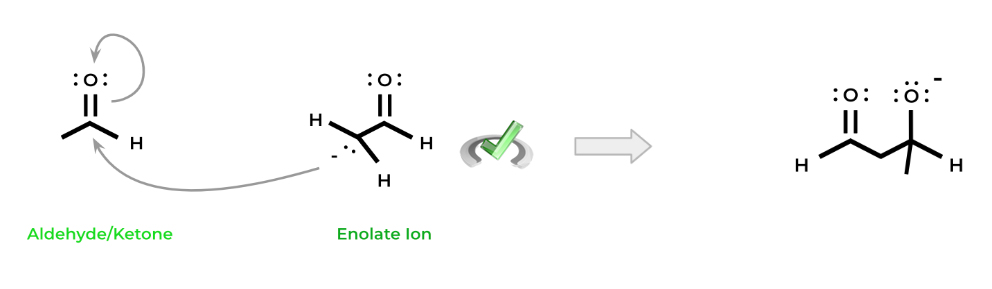
This attack mimics the nucleophilic addition reaction we covered above: the nucleophilic carbanion of the enolate attacks the carbonyl of the aldehyde/ketone, causing the movement of an electron pair onto the oxygen.
The oxygen is then protonated, resulting in the formation of an alcohol. Hence, we get the term aldol: the “-al” portion refers to the aldehyde while the “-ol” term refers to the alcohol. Note that this reaction can also occur with ketones despite the name.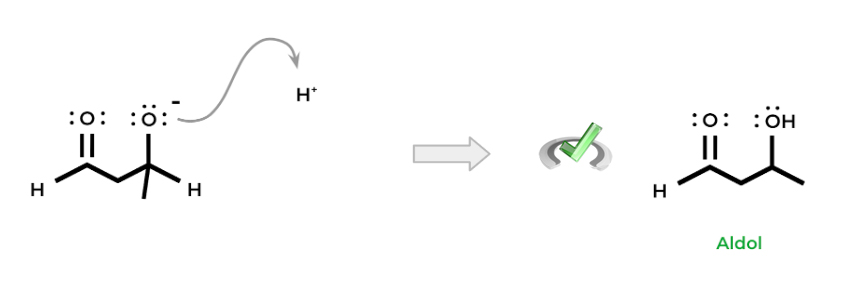
Notice how overall, in the reaction, we added a carbon-carbon bond which is one of the main benefits of aldol addition.
In addition, if this reaction occurs in the presence of heat, we can get what’s called an aldol condensation which releases a water molecule per its name. This results in the formation of a carbon-carbon double bond while the hydroxyl group is also removed.
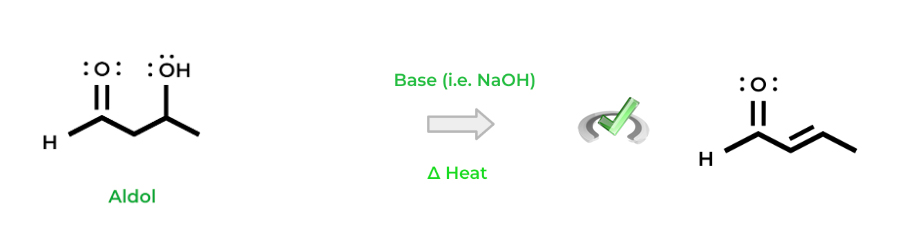
Full Study Notes : Aldol Addition and Condensation
For more in-depth content review on aldol additions and condensations, check out these detailed lesson notes created by top MCAT scorers.
Important Definitions and Key Terms
Below are some high yield definitions and key terms to refer to when reviewing concepts and ideas about aldehydes and ketones in organic chemistry!
Term | Definition |
|---|---|
Aldehyde | An organic molecule characterized by a carbonyl group with carbonyl carbon being attached to a hydrogen atom and another carbon based molecule |
Ketone | An organic molecule characterized by a carbonyl group with the carbonyl carbon being attached to 2 carbon based molecules |
Geminal Diol | An alcohol where 2 hydroxyl groups are attached to the same carbon; Can be formed via the hydration of an aldehyde/ketone |
Hemiacetal/Hemiketal | The intermediate point between acetaldehyde/ketone and an acetal/ketal; Can be formed by the nucleophilic addition of 1 equivalent of alcohol |
Acetal/Ketal | Formed via the nucleophilic addition of 2 equivalents of alcohol; Similar structure to geminal diols except hydroxyl groups are replaced by 2 alkoxide groups |
Cyanohydrins | Organic molecule which can be formed via the nucleophilic addition of a cyanide ion to an aldehyde/ketone |
Enolate Ion | Anionic compound that is generated by the deprotonation of an 𝜶-hydrogen from an aldehyde/ketone |
𝜶-Hydrogen | Refers to one of the hydrogen located on the 𝜶-carbon which is the next adjacent carbon to the carbonyl carbon |
Keto-Enol Tautomerism | State of equilibrium which exists betweens the “keto” forms of aldehydes/ketones and their “enol” counterparts |
Enol | Characterized by the presence of a carbon-carbon double bond and an alcohol; Exist in equilibrium with the molecule’s “keto” form |
Aldol | Characterized by the presence of an alcohol on an aldehyde or ketone; Can be formed by the nucleophilic attack of an enolate ion on its corresponding aldehyde/ketone |
Additional FAQs - Aldehydes and Ketones on the MCAT
What is the Relationship Between Aldehydes and Ketones? – MCAT
Can Ketones Be Oxidized? – MCAT
What is an Acetal? – MCAT
What Functional Groups Do You Need to Know for the MCAT?
Additional Reading -- Organic Chemistry Topics:
- Alcohols and Ethers on the MCAT
- Bonding on the MCAT
- Carboxylic Acids and Derivatives on the MCAT
- Isomers on the MCAT
- Laboratory Techniques and Separations on the MCAT
- Nitrogen Containing Compounds on the MCAT
- Phosphorus Containing Compounds on the MCAT
- Organic Chemistry Nomenclature on the MCAT
- Nucleophiles and Electrophiles on the MCAT
- Spectroscopy on the MCAT
- Redox Reactions Organic Chemistry on the MCAT







 To help you achieve your goal MCAT score, we take turns hosting these
To help you achieve your goal MCAT score, we take turns hosting these 
